Parkinson's disease variant detection and disclosure: PD GENEration, a North American study
- PMID: 39074992
- PMCID: PMC11292896
- DOI: 10.1093/brain/awae142
Parkinson's disease variant detection and disclosure: PD GENEration, a North American study
Abstract
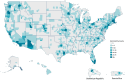
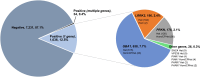
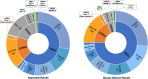
Variants in seven genes (LRRK2, GBA1, PRKN, SNCA, PINK1, PARK7 and VPS35) have been formally adjudicated as causal contributors to Parkinson's disease; however, individuals with Parkinson's disease are often unaware of their genetic status since clinical testing is infrequently offered. As a result, genetic information is not incorporated into clinical care, and variant-targeted precision medicine trials struggle to enrol people with Parkinson's disease. Understanding the yield of genetic testing using an established gene panel in a large, geographically diverse North American population would help patients, clinicians, clinical researchers, laboratories and insurers better understand the importance of genetics in approaching Parkinson's disease. PD GENEration is an ongoing multi-centre, observational study (NCT04057794, NCT04994015) offering genetic testing with results disclosure and genetic counselling to those in the US (including Puerto Rico), Canada and the Dominican Republic, through local clinical sites or remotely through self-enrolment. DNA samples are analysed by next-generation sequencing including deletion/duplication analysis (Fulgent Genetics) with targeted testing of seven major Parkinson's disease-related genes. Variants classified as pathogenic/likely pathogenic/risk variants are disclosed to all tested participants by either neurologists or genetic counsellors. Demographic and clinical features are collected at baseline visits. Between September 2019 and June 2023, the study enrolled 10 510 participants across >85 centres, with 8301 having received results. Participants were: 59% male; 86% White, 2% Asian, 4% Black/African American, 9% Hispanic/Latino; mean age 67.4 ± 10.8 years. Reportable genetic variants were observed in 13% of all participants, including 18% of participants with one or more 'high risk factors' for a genetic aetiology: early onset (<50 years), high-risk ancestry (Ashkenazi Jewish/Basque/North African Berber), an affected first-degree relative; and, importantly, in 9.1% of people with none of these risk factors. Reportable variants in GBA1 were identified in 7.7% of all participants; 2.4% in LRRK2; 2.1% in PRKN; 0.1% in SNCA; and 0.2% in PINK1, PARK7 or VPS35 combined. Variants in more than one of the seven genes were identified in 0.4% of participants. Approximately 13% of study participants had a reportable genetic variant, with a 9% yield in people with no high-risk factors. This supports the promotion of universal access to genetic testing for Parkinson's disease, as well as therapeutic trials for GBA1 and LRRK2-related Parkinson's disease.
Keywords: GBA1; LRRK2; Parkinson’s disease; clinical trials; genetic counselling; genetic testing.
© The Author(s) 2024. Published by Oxford University Press on behalf of the Guarantors of Brain.
Conflict of interest statement
L.C. and J.S. received partial funding of salaries from the Michael J. Fox Foundation (MJFF) and the Parkinson’s Foundation; J.V. received partial funding of salaries from MJFF and the Parkinson’s Foundation and received travel funding for the Movement Disorders Society International Congress, Madrid, Spain, September 2022, abstract and poster presentation ‘PD GENEration Clinical Phase: Genetic Diagnostic Yield and Clinical Characteristics’ from the Parkinson’s Foundation and paid to Indiana University School of Medicine; T.F. received funding of grants from Parkinson’s Foundation for acting as a Steering Committee Member and received funding for grants/contracts from Parkinson’s Foundation; A.H. is a research advocate and received funding of grants from Parkinson’s Foundation for acting as a Steering Committee Member; K.S.M. received funding of grants from Parkinson’s Foundation for acting as a Steering Committee Member, receives funding of grants from NIH, MJFF, CHDI, HSG, HDSA, Prilenia, Novartis, Roche and Springer and receives consulting fees from Novartis and funding for leadership on the Enroll HD Oversight Committee (CHDI) and HSG steering committee; I.F.M. received funding of grants from the Parkinson’s Foundation for acting as a Steering Committee Member and receives funding of grants/conference support from MJFF, ASAP, NIH and Cleveland Clinic Foundation; N.E.M. received funding of grants from the Parkinson’s Foundation for acting as a Steering Committee Member, receives funding of grants from GP2 Monogenic Network, receives payment/honoraria for MDS lectures and received support for attending the MDS congress in Madrid, September 2022; M.A.N. received compensation for work as a Steering Committee member for studies conducted by Bial and Neurocrine, for consulting work from Roche, Novartis and Uniqure, grant funding from CHDI and HDSA and received funding of grants from the Parkinson’s Foundation for acting as a Steering Committee Member, received payment for presentation at Parkinson’s Foundation and Parkinson Study Group conferences, received support for attending Parkinson Study Group conference and is the Chair of Genetics and environment Working Group for the Parkinson Study Group; M.A.S. received funding of grants from the Parkinson’s Foundation for acting as a Steering Committee Member, receives funding of grants or conference support from NIH, MJFF, Farmer Family Foundation, Sergey Brin Family Foundation, American Parkinson’s Disease Association, Cure Parkinson’s, Parkinson’s Foundation, Harvard School of Public Health (NIH, DoD), GSK and GE Healthcare, receives consulting fees through the Parkinson Study Group for PSG advisory services [including to Bial, Biogen (LUMA/LIGHTHOUSE trials global SC), UCB (ORHCESTRA trial PSG SC)] and through Sutter Health (NIA), Northwestern University (NINDS), Cure Parkinson’s for Steering Committee services (for TOPAZ and SPARX3), International Linked Clinical Trial Committee (CP), participates on an Alzheimer’s drug trial Data Monitoring Committee, provides services for Eli Lilly & Co. and is Chair, Executive Committee for Parkinson Study Group; T.S. received funding of grants from the Parkinson’s Foundation for acting as a Steering Committee Member, receives funding of grants from Amneal, Biogen, Roche, Neuroderm, Sanofi, Prevail and UCB, is an investigator for NINDS, MJFF, Parkinson’s Foundation studies, receives consulting fees from 4D Pharma, Acadia, AcureX, AskBio, Amneal, Blue Rock Therapeutics, Caraway Therapeutics, Critical Path for Parkinson's Consortium (CPP), Denali, MJFF, Neuroderm, Sanofi, Sinopia, Sunovion, Roche, Takeda, UCB, Vanqua Bio and Voyager and is on the advisory board for Acadia, AcureX, AskBio, Amneal, Denali, Sunovion and Roche and the Scientific Advisory Board for 4D Pharma, Neuroderm, Sanofi and UCB; A.M.W. received funding of grants from the Parkinson’s Foundation for acting as a Steering Committee Member and receives funding of grants from NIA/NIH, Roche/Genentech, Biogen and their institution, participates on Data Safety Monitoring Board or Advisory Board for Ono Pharmaceuticals and Amylyx Pharmaceuticals; S.B. receives funding of a grant through institution for acting as a site principal investigator for PD GENEration; H.H.F. receives funding of a grant through institution for acting as a site principal investigator for PD GENEration, receives funding of grants from Biogen, MJFF, NINDS and Roche, receives consulting fees for the Parkinson Study Group, Cerevel, Amneal, AbbVie, receives royalties as a book author from Springer Publishing, is Co-Chair of the PSG Executive Committee, is a member of the Scientific Advisory Board for CCXDP and receives payment as Editor-in-Chief of Parkinsonism and Related Disorders from Elsevier; I.L. receives funding of a grant through institution for acting as a site principal investigator for PD GENEration, receives funding from the National Institutes of Health grants: 2R01AG038791-06A, U01NS100610, U01NS80818, R25NS098999; U19 AG063911-1 and 1R21NS114764-01A1, MJFF, the Parkinson Foundation, Lewy Body Association, CurePSP, Roche, Abbvie, Biogen, Centogene. EIP-Pharma, Biohaven Pharmaceuticals, Novartis and United Biopharma SRL and UCB and is on the Scientific Advisory Board for Amydis and on the Scientific Advisory Board of the Rossy PSP Program at the University of Toronto; H.A.S. receives funding of a grant through institution for acting as a site principal investigator for PD GENEration, receives grants from UCB Pharma, Transposon Therapeutics, Jazz Pharmaceuticals, Barrow Neurological Foundation, MJFF and NINDS/NIH and receives consulting fees from Sage/Biogen, AbbVie and Parkinson Study Group/NQ; C.S. receives funding of a grant through institution for acting as a site principal investigator for PD GENEration, receives grants from Pharma Two B, TEVA Pharmaceuticals, Amneal Pharmaceuticals, Revance Therapeutics and Sunovion Pharmaceuticals; T.F.T. receives funding of a grant through institution for acting as a site principal investigator for PD GENEration and receives grants from MJFF and NIH; N.V.A. receives funding of a grant through institution for acting as a site principal investigator for PD GENEration and receives grants from MJFF and NIH; R.C.V. receives funding of a grant through institution for acting as a site principal investigator for PD GENEration; L.K. receives funding of a grant through institution for acting as a site principal investigator for PD GENEration; J.F.Q. receives funding of a grant through institution for acting as a site principal investigator for PD GENEration, receives funding for acting as support to their institution as an LBDA RCOE and serves on DSMB for NIH and for Travere Pharmaceuticals; P.D.H. received partial funding of salary from the Parkinson’s Foundation and receives funding for acting on the PD GENE Latino Advisory Committee; Y.M. is an employee of Fulgent Genetics; S.P.S. received support for attending meetings from Illumina, Inc; S.C.R. is employed by the Parkinson’s Foundation; K.G.G. is employed by the Parkinson’s Foundation; A.N. is employed by the Parkinson’s Foundation; J.C.B. is employed by the Parkinson’s Foundation and receives funding for grants through their institution from NIH; R.N.A. received grants from the Parkinson’s Foundation for acting as a Steering Committee Member, receives funding from NIH, Department of Defense, MJFF and the Silverstein Foundation for GBA/Parkinson’s disease. He received consulting fees from Biogen, Biohaven, Capsida, Gain Therapeutics, Sanofi, Servier, Takeda and Vanqua bio. The other authors report no competing interests.
Figures
Comment in
-
Clinical genetic testing in Parkinson's disease should become part of routine patient care.Brain. 2024 Aug 1;147(8):2595-2597. doi: 10.1093/brain/awae181. Brain. 2024. PMID: 39087915
Similar articles
-
Relevance of genetic testing in the gene-targeted trial era: the Rostock Parkinson's disease study.Brain. 2024 Aug 1;147(8):2652-2667. doi: 10.1093/brain/awae188. Brain. 2024. PMID: 39087914 Free PMC article.
-
Defining the optimum strategy for identifying adults and children with coeliac disease: systematic review and economic modelling.Health Technol Assess. 2022 Oct;26(44):1-310. doi: 10.3310/ZUCE8371. Health Technol Assess. 2022. PMID: 36321689 Free PMC article.
-
Adenosine Deaminase Deficiency.2006 Oct 3 [updated 2024 Mar 7]. In: Adam MP, Feldman J, Mirzaa GM, Pagon RA, Wallace SE, Amemiya A, editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993–2025. 2006 Oct 3 [updated 2024 Mar 7]. In: Adam MP, Feldman J, Mirzaa GM, Pagon RA, Wallace SE, Amemiya A, editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993–2025. PMID: 20301656 Free Books & Documents. Review.
-
RETRACTED: Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial.Int J Antimicrob Agents. 2020 Jul;56(1):105949. doi: 10.1016/j.ijantimicag.2020.105949. Epub 2020 Mar 20. Int J Antimicrob Agents. 2020. Retraction in: Int J Antimicrob Agents. 2025 Jan;65(1):107416. doi: 10.1016/j.ijantimicag.2024.107416. PMID: 32205204 Free PMC article. Retracted. Clinical Trial.
-
Hemophilia B.2000 Oct 2 [updated 2024 Jun 6]. In: Adam MP, Feldman J, Mirzaa GM, Pagon RA, Wallace SE, Amemiya A, editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993–2025. 2000 Oct 2 [updated 2024 Jun 6]. In: Adam MP, Feldman J, Mirzaa GM, Pagon RA, Wallace SE, Amemiya A, editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993–2025. PMID: 20301668 Free Books & Documents. Review.
Cited by
-
Clinical genetic testing in Parkinson's disease should become part of routine patient care.Brain. 2024 Aug 1;147(8):2595-2597. doi: 10.1093/brain/awae181. Brain. 2024. PMID: 39087915
-
The LRRK2 p.L1795F variant causes Parkinson's disease in the European population.Res Sq [Preprint]. 2024 Sep 20:rs.3.rs-4772543. doi: 10.21203/rs.3.rs-4772543/v1. Res Sq. 2024. PMID: 39372927 Free PMC article. Preprint.
References
-
- Bloem BR, Okun MS, Klein C. Parkinson’s disease. Lancet. 2021;397:2284–2293. - PubMed
-
- Lesage S, Brice A. Parkinson’s disease: From monogenic forms to genetic susceptibility factors. Hum Mol Genet. 2009;18(R1):R48–R59. - PubMed
-
- Kim CY, Alcalay RN. Genetic forms of Parkinson’s disease. Semin Neurol. 2017;37:135–146. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

