Cell-cell transfer of adaptation traits benefits kin and actor in a cooperative microbe
- PMID: 39012831
- PMCID: PMC11287280
- DOI: 10.1073/pnas.2402559121
Cell-cell transfer of adaptation traits benefits kin and actor in a cooperative microbe
Abstract
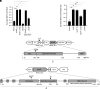
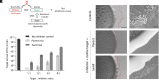
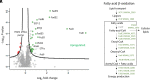
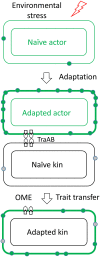
Microbes face many physical, chemical, and biological insults from their environments. In response, cells adapt, but whether they do so cooperatively is poorly understood. Here, we use a model social bacterium, Myxococcus xanthus, to ask whether adapted traits are transferable to naïve kin. To do so we isolated cells adapted to detergent stresses and tested for trait transfer. In some cases, strain-mixing experiments increased sibling fitness by transferring adaptation traits. This cooperative behavior depended on a kin recognition system called outer membrane exchange (OME) because mutants defective in OME could not transfer adaptation traits. Strikingly, in mixed stressed populations, the transferred trait also benefited the adapted (actor) cells. This apparently occurred by alleviating a detergent-induced stress response in kin that otherwise killed actor cells. Additionally, this adaptation trait when transferred also conferred resistance against a lipoprotein toxin delivered to targeted kin. Based on these and other findings, we propose a model for stress adaptation and how OME in myxobacteria promotes cellular cooperation in response to environmental stresses.
Keywords: Myxococcus xanthus; cooperation; detergent resistance; kin recognition; outer membrane exchange.
Conflict of interest statement
Competing interests statement:The authors declare no competing interest.
Figures



Similar articles
-
Healthcare workers' informal uses of mobile phones and other mobile devices to support their work: a qualitative evidence synthesis.Cochrane Database Syst Rev. 2024 Aug 27;8(8):CD015705. doi: 10.1002/14651858.CD015705.pub2. Cochrane Database Syst Rev. 2024. PMID: 39189465 Free PMC article.
-
Public engagement to refine a whole-school intervention to promote adolescent mental health.Public Health Res (Southampt). 2024 Dec 4:1-22. doi: 10.3310/JWGT4863. Online ahead of print. Public Health Res (Southampt). 2024. PMID: 39636228
-
Using Experience Sampling Methodology to Capture Disclosure Opportunities for Autistic Adults.Autism Adulthood. 2023 Dec 1;5(4):389-400. doi: 10.1089/aut.2022.0090. Epub 2023 Dec 12. Autism Adulthood. 2023. PMID: 38116059 Free PMC article.
-
Depressing time: Waiting, melancholia, and the psychoanalytic practice of care.In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. PMID: 36137063 Free Books & Documents. Review.
-
Interventions to reduce harm from continued tobacco use.Cochrane Database Syst Rev. 2016 Oct 13;10(10):CD005231. doi: 10.1002/14651858.CD005231.pub3. Cochrane Database Syst Rev. 2016. PMID: 27734465 Free PMC article. Review.
References
-
- Hamilton W. D., The genetical evolution of social behaviour. I. J. Theor. Biol. 7, 1–16 (1964). - PubMed
-
- Strassmann J. E., Gilbert O. M., Queller D. C., Kin discrimination and cooperation in microbes. Annu. Rev. Microbiol. 65, 349–367 (2011). - PubMed
-
- Velicer G. J., Kroos L., Lenski R. E., Developmental cheating in the social bacterium Myxococcus xanthus. Nature 404, 598–601 (2000). - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

