Efficacy and safety of margetuximab plus chemotherapy vs. trastuzumab plus chemotherapy in Chinese patients with pretreated HER2-positive advanced metastatic breast cancer: results from a randomized, open-label, multicenter, phase II bridging study
- PMID: 38751523
- PMCID: PMC11093073
- DOI: 10.21037/tbcr-22-35
Efficacy and safety of margetuximab plus chemotherapy vs. trastuzumab plus chemotherapy in Chinese patients with pretreated HER2-positive advanced metastatic breast cancer: results from a randomized, open-label, multicenter, phase II bridging study
Abstract
Background: Trastuzumab is the recommended first-line treatment for human epidermal growth factor receptor 2 (HER2)-positive metastatic breast cancer (MBC) patients in China, but therapeutic resistance to trastuzumab and other early-line treatments is common and late-line treatment options are limited. Derived from the same murine precursor antibody, margetuximab has enhanced anti-tumor activity compared with trastuzumab and may be an effective late-line treatment. However, data regarding the use of margetuximab in pre-treated Chinese patients are scarce. This study aimed to evaluate the efficacy and safety of margetuximab plus chemotherapy vs. trastuzumab plus chemotherapy in Chinese patients, and to determine whether the results are consistent with the clinical benefit of margetuximab observed in the pivotal global phase III study.
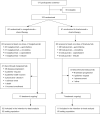
Methods: In this randomized, open-label, multicenter, phase II bridging study, eligible Chinese patients pretreated with ≥2 lines of anti-HER2 therapies were randomized 1:1 by stratified block randomization to margetuximab (15 mg/kg over at least 120 minutes) or trastuzumab (6 mg/kg over at least 30 minutes), each administered intravenously once every 21-day cycle and plus chemotherapy. Disease assessment was conducted once every two treatment cycles (6 weeks ± 7 days). The primary endpoint was progression-free survival (PFS) by blinded independent central review (BICR). Secondary endpoints included overall survival (OS), investigator-assessed PFS, objective response rate (ORR), duration of response (DoR), clinical benefit rate (CBR), and the incidence and severity of treatment-emergent adverse events (TEAEs).
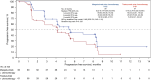
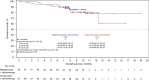
Results: Between February 4, 2020 and February 23, 2021, 123 patients were randomized to the margetuximab (n=62) and trastuzumab (n=61) arms. Among them, 15 and 7 patients, respectively, were still on study treatment as of data cut-off (September 3, 2021). Overall, 99.2% were female, median age was 53 years old. All patients were pretreated with trastuzumab, and 83.7% and 25.2%, respectively, were pretreated with tyrosine kinase inhibitors (TKIs) and pertuzumab. Baseline characteristics were numerically balanced between arms. BICR-assessed median PFS (mPFS) was 5.5 months in the margetuximab arm and 4.1 months in the trastuzumab arm, with a hazard ratio (HR) of 0.69 [95% confidence interval (CI): 0.43-1.12], which met the consistency criterion (HR <0.88) for bridging success. Median investigator-assessed PFS was 5.5 months in the margetuximab arm and 4.0 months in the trastuzumab arm (HR =0.63; 95% CI: 0.41-0.96). Median OS (mOS) was not yet reached. Both ORR and CBR were greater in the margetuximab arm (25.5% vs. 12.5%; 32.7% vs. 14.3%). Safety results were numerically comparable between the two arms. Anti-HER2 treatment-related infusion-related reactions (IRRs) were more common in the margetuximab arm than in the trastuzumab arm (12.9% vs. 1.7%). All IRRs could be resolved.
Conclusions: Margetuximab was effective and well-tolerated in this study, supporting its clinical use in pretreated HER2-positive MBC patients in China.
Trial registration: ClinicalTrials.gov Identifier: NCT04262804.
Keywords: Chinese; human epidermal growth factor receptor 2-positive (HER2-positive); margetuximab; metastatic breast cancer (MBC).
2022 Translational Breast Cancer Research. All rights reserved.
Conflict of interest statement
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tbcr.amegroups.com/article/view/10.21037/tbcr-22-35/coif). This work was sponsored by Zai Lab (Shanghai) Co., Ltd. ZJ serves as an Editor-in-Chief of Translational Breast Cancer Research. QZ, XW, HW, YY, TS, SW, and YP serve as unpaid editorial board members of Translational Breast Cancer Research from Mar 2022 to Feb 2024. LY and YX are employees of Zai Lab (Shanghai) Co., Ltd. and hold stock and stock options of Zai Lab (Shanghai) Co., Ltd. The other authors have no conflicts of interest to declare.
Figures

Similar articles
-
Trastuzumab deruxtecan in patients with metastatic non-small-cell lung cancer (DESTINY-Lung01): primary results of the HER2-overexpressing cohorts from a single-arm, phase 2 trial.Lancet Oncol. 2024 Apr;25(4):439-454. doi: 10.1016/S1470-2045(24)00064-0. Lancet Oncol. 2024. PMID: 38547891 Clinical Trial.
-
Comparison of efficacy and safety of PD-1/PD-L1 combination therapy in first-line treatment of advanced NSCLC: an updated systematic review and network meta-analysis.Clin Transl Oncol. 2024 Oct;26(10):2488-2502. doi: 10.1007/s12094-024-03442-3. Epub 2024 Apr 16. Clin Transl Oncol. 2024. PMID: 38625495
-
Oral budesonide for induction of remission in ulcerative colitis.Cochrane Database Syst Rev. 2015 Oct 26;2015(10):CD007698. doi: 10.1002/14651858.CD007698.pub3. Cochrane Database Syst Rev. 2015. PMID: 26497719 Free PMC article. Review.
-
Falls prevention interventions for community-dwelling older adults: systematic review and meta-analysis of benefits, harms, and patient values and preferences.Syst Rev. 2024 Nov 26;13(1):289. doi: 10.1186/s13643-024-02681-3. Syst Rev. 2024. PMID: 39593159 Free PMC article.
-
Impact of residual disease as a prognostic factor for survival in women with advanced epithelial ovarian cancer after primary surgery.Cochrane Database Syst Rev. 2022 Sep 26;9(9):CD015048. doi: 10.1002/14651858.CD015048.pub2. Cochrane Database Syst Rev. 2022. PMID: 36161421 Free PMC article. Review.
Cited by
-
A real-world study of treatment sequences and second-line clinical outcomes in patients with HER2-positive metastatic breast cancer in US community practice.Int J Clin Oncol. 2024 Jun;29(6):780-789. doi: 10.1007/s10147-024-02492-5. Epub 2024 Mar 25. Int J Clin Oncol. 2024. PMID: 38528295 Free PMC article.
References
-
- He J, Chen WQ, Li N, et al. China guideline for the screening and early detection of female breast cancer (2021, Beijing). Zhonghua Zhong Liu Za Zhi 2021;43:357-82. - PubMed
-
- Breast Cancer Expert Committee of China Anti-Cancer Association . Guidelines and Standards for the Diagnosis and Treatment of Breast Cancer by the China Anti-Cancer Association (2021 edition). China Oncology 2021;31:954-1040.
-
- Breast Cancer Expert Committee of National Cancer Quality Control Center. Breast Cancer Expert Committee of China Anti-Cancer Association ; Cancer Drug Clinical Research Committee of China Anti-Cancer Association. Guidelines for clinical diagnosis and treatment of advanced breast cancer in China (2020 Edition). Zhonghua Zhong Liu Za Zhi 2020;42:781-97. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
