Long-Term Comparative Efficacy and Safety of Risdiplam and Nusinersen in Children with Type 1 Spinal Muscular Atrophy
- PMID: 38705943
- PMCID: PMC11133132
- DOI: 10.1007/s12325-024-02845-6
Long-Term Comparative Efficacy and Safety of Risdiplam and Nusinersen in Children with Type 1 Spinal Muscular Atrophy
Abstract
Introduction: Spinal muscular atrophy (SMA) is a severe genetic neuromuscular disease characterized by a loss of motor neurons and progressive muscle weakness. Children with untreated type 1 SMA never sit independently and require increasing levels of ventilatory support as the disease progresses. Without intervention, and lacking ventilatory support, death typically occurs before the age of 2 years. There are currently no head-to-head trials comparing available treatments in SMA. Indirect treatment comparisons are therefore needed to provide information on the relative efficacy and safety of SMA treatments for healthcare decision-making.
Methods: The long-term efficacy and safety of risdiplam versus nusinersen in children with type 1 SMA was evaluated using indirect treatment comparison methodology to adjust for differences between population baseline characteristics, to reduce any potential bias in the comparative analysis. An unanchored matching-adjusted indirect comparison was conducted using risdiplam data from 58 children in FIREFISH (NCT02913482) and published aggregate nusinersen data from 81 children obtained from the ENDEAR (NCT02193074) and SHINE (NCT02594124) clinical trials with at least 36 months of follow-up.
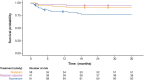
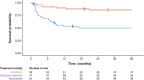
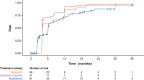
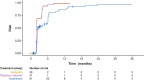
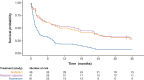
Results: Children with type 1 SMA treated with risdiplam had a 78% reduction in the rate of death, an 81% reduction in the rate of death or permanent ventilation, and a 57% reduction in the rate of serious adverse events compared with children treated with nusinersen. Children treated with risdiplam also had a 45% higher rate of achieving a Hammersmith Infant Neurological Examination, Module 2 motor milestone response and a 186% higher rate of achieving a ≥ 4-point improvement in Children's Hospital of Philadelphia Infant Test of Neuromuscular Disorders compared with children treated with nusinersen.
Conclusion: Long-term data supported risdiplam as a superior alternative to nusinersen in children with type 1 SMA. Video abstract available for this article. Video abstract (MP4 184542 KB).
Keywords: Clinical trial; Indirect treatment comparison; MAIC; Matching-adjusted indirect comparison; Neuromuscular disease; Nusinersen; Propensity score matching; Risdiplam; SMA; Spinal muscular atrophy.
Plain language summary
Risdiplam and nusinersen are two approved treatments for patients with type 1 spinal muscular atrophy (SMA). There are currently no head-to-head trials that compare the outcomes of these treatments in patients. This study conducted a statistical comparison of the efficacy and safety of risdiplam and nusinersen in children with type 1 SMA who received treatment for at least 36 months. Risdiplam data were collected from 58 children who participated in the FIREFISH trial (NCT02913482). Published combined data were collected from 81 children treated with nusinersen who participated in the ENDEAR (NCT02193074) and SHINE (NCT02594124) trials. Outcomes from the two studies were compared using matching-adjusted indirect comparison (MAIC) methodology. MAIC adjusts for differences in baseline characteristics between patients in two trials to make the populations more similar and reduce bias in the comparison. Results suggested that children with type 1 SMA treated with risdiplam had a 78% reduction in the rate of death and an 81% reduction in the rate of death or permanent ventilation compared with children treated with nusinersen. With risdiplam, children also had a higher rate of achieving motor function responses, and a longer time to the first serious adverse event compared with children treated with nusinersen. These results support risdiplam as a superior alternative to nusinersen in children with type 1 SMA over 36 months of follow-up. Access to long-term data beyond 36 months would allow for additional indirect comparisons between SMA treatments. These comparisons are key to guiding treatment decision-making in the absence of head-to-head trials.
© 2024. The Author(s).
Conflict of interest statement
The SLR and data extraction were conducted by Bridge Medical Consulting Ltd., London, UK and funded by F. Hoffmann-La Roche Ltd., Basel, Switzerland. ITC analyses were designed by Visible Analytics, UK, funded by F. Hoffmann-La Roche Ltd. Christos Kokaliaris and Julian Nam are employees of F. Hoffmann-La Roche Ltd. Julian Nam owns stocks in Roche. Rachel Evans is a former employee of Visible Analytics Ltd., which designed the analysis and received consultancy fees and expenses from F. Hoffmann-La Roche Ltd. Neil Hawkins and David Alexander Scott are partners/employees of Visible Analytics Ltd., which designed the analysis and received consultancy fees and expenses from F. Hoffmann-La Roche Ltd. Anadi Mahajan is an employee of Bridge Medical Consulting Ltd., which conducted this SLR and received consultancy fees and expenses from F. Hoffmann-La Roche Ltd. C Simone Sutherland was employed by F. Hoffmann-La Roche Ltd and owns stocks in Roche. Gautam Sajeev is an employee of Analysis Group, Inc. which received consulting fees from Genentech Inc. (a member of the Roche group) for this research. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Figures
Similar articles
-
Drug treatment for spinal muscular atrophy type I.Cochrane Database Syst Rev. 2019 Dec 11;12(12):CD006281. doi: 10.1002/14651858.CD006281.pub5. Cochrane Database Syst Rev. 2019. PMID: 31825542 Free PMC article.
-
Risdiplam-Treated Infants with Type 1 Spinal Muscular Atrophy versus Historical Controls.N Engl J Med. 2021 Jul 29;385(5):427-435. doi: 10.1056/NEJMoa2102047. N Engl J Med. 2021. PMID: 34320287
-
How does risdiplam compare with other treatments for Types 1-3 spinal muscular atrophy: a systematic literature review and indirect treatment comparison.J Comp Eff Res. 2022 Apr;11(5):347-370. doi: 10.2217/cer-2021-0216. Epub 2022 Jan 18. J Comp Eff Res. 2022. PMID: 35040693 Review.
-
Survival, Motor Function, and Motor Milestones: Comparison of AVXS-101 Relative to Nusinersen for the Treatment of Infants with Spinal Muscular Atrophy Type 1.Adv Ther. 2019 May;36(5):1164-1176. doi: 10.1007/s12325-019-00923-8. Epub 2019 Mar 16. Adv Ther. 2019. PMID: 30879249 Free PMC article.
-
An updated systematic review on spinal muscular atrophy patients treated with nusinersen, onasemnogene abeparvovec (at least 24 months), risdiplam (at least 12 months) or combination therapies.Eur J Paediatr Neurol. 2024 Jul;51:84-92. doi: 10.1016/j.ejpn.2024.06.004. Epub 2024 Jun 17. Eur J Paediatr Neurol. 2024. PMID: 38905882 Review.
Cited by
-
Clinical perspectives: Treating spinal muscular atrophy.Mol Ther. 2024 Aug 7;32(8):2489-2504. doi: 10.1016/j.ymthe.2024.06.020. Epub 2024 Jun 18. Mol Ther. 2024. PMID: 38894541 Review.
References
-
- Mercuri E, Sumner CJ, Muntoni F, Darras BT, Finkel RS. Spinal muscular atrophy. Nat Rev Dis Primers. 2022;8(1):52. - PubMed
-
- Lefebvre S, Burglen L, Reboullet S, et al. Identification and characterization of a spinal muscular atrophy-determining gene. Cell. 1995;80(1):155–165. - PubMed
-
- Munsat TL, Davies KE. International SMA consortium meeting. (26–28 June 1992, Bonn, Germany) Neuromuscul Disord. 1992;2(56):423–428. - PubMed
-
- Kaneko K, Arakawa R, Urano M, Aoki R, Saiito K. Relationships between long-term observations of motor milestones and genotype analysis results in childhood-onset Japanese spinal muscular atrophy patients. Brain Dev. 2017;39(9):763–773. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

