Switching from Nusinersen to Risdiplam: A Croatian Real-World Experience on Effectiveness and Safety
- PMID: 38540986
- PMCID: PMC10971370
- DOI: 10.3390/jpm14030244
Switching from Nusinersen to Risdiplam: A Croatian Real-World Experience on Effectiveness and Safety
Abstract
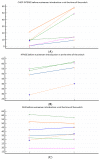
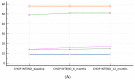
(1) Background: Data on combination or sequential treatment of spinal muscular atrophy (SMA) with disease-modifying drugs (DMDs) are missing and the latter field is poorly understood. The currently available data of patients on risdiplam previously treated with nusinersen are coming from exploratory research mainly focused on safety. Our aim was to investigate the real-world effectiveness (hypothesising non-inferiority) and safety profile of risdiplam in a paediatric-and-adult nusinersen-risdiplam spinal muscular atrophy switch cohort. (2) Methods: A retrospective and anonymous collection of relevant demographic and clinical data for all Croatian SMA patients switched from nusinersen to risdiplam up to September 2023 (reimbursed by Croatian Health Insurance Fund-CHIF) was performed using the CHIF database and associated reimbursement documentation. Patients were included in effectiveness and safety analysis if they met the following inclusion criteria: (i) risdiplam was reimbursed by the CHIF; (ii) the patient received at least six doses of nusinersen before the switch to risdiplam; (iii) there was no relevant pause between the latter disease-modifying drugs; (iv) availability of all prespecified studied data and parameters. (3) Results: In total, 17 patients met the inclusion criteria (58.9% female; median age 12.75 (3.0-44.5) years). In our 'switch' cohort, we demonstrated a non-inferiority of risdiplam to nusinersen in the SMA 1 (+1.0 in CHOP INTEND; p = 0.067), SMA 3p (+0.7 in HFMSE; p = 0.897), and SMA 3a (+0.8 in RHS; p = 0.463) subpopulations, during a one-year follow-up period. There were no reports on respiratory function worsening, feeding worsening, and no lethal events. No new safety concerns were identified, except for the weight gain that arose as a new potential adverse drug reaction 'signal' in two patients. (4) Conclusions: We have reported pivotal real-world findings on switching SMA patients from nusinersen to risdiplam and demonstrated its effectiveness (non-inferiority), safety, and tolerability in a heterogenous paediatric-and-adult 'switch' cohort; this will further increase the quality and standards of care as well as safety of a notable portion of SMA patients, especially for those who demand the switch from nusinersen to other DMDs for clinical or personal reasons.
Keywords: effectiveness; nusinersen; real-world data; risdiplam; spinal muscular atrophy.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Effectiveness of Nusinersen in Type 1, 2 and 3 Spinal Muscular Atrophy: Croatian Real-World Data.J Clin Med. 2023 Apr 13;12(8):2839. doi: 10.3390/jcm12082839. J Clin Med. 2023. PMID: 37109175 Free PMC article.
-
Long-Term Comparative Efficacy and Safety of Risdiplam and Nusinersen in Children with Type 1 Spinal Muscular Atrophy.Adv Ther. 2024 Jun;41(6):2414-2434. doi: 10.1007/s12325-024-02845-6. Epub 2024 May 5. Adv Ther. 2024. PMID: 38705943 Free PMC article.
-
Health Care Resource Utilization and Costs for Patients with Spinal Muscular Atrophy: Findings from a Retrospective US Claims Database Analysis.Adv Ther. 2023 Oct;40(10):4589-4605. doi: 10.1007/s12325-023-02621-y. Epub 2023 Aug 16. Adv Ther. 2023. PMID: 37587305 Free PMC article.
-
Safety and Efficacy of Nusinersen and Risdiplam for Spinal Muscular Atrophy: A Systematic Review and Meta-Analysis of Randomized Controlled Trials.Brain Sci. 2023 Oct 7;13(10):1419. doi: 10.3390/brainsci13101419. Brain Sci. 2023. PMID: 37891788 Free PMC article. Review.
-
Mid- and long-term (at least 12 months) follow-up of patients with spinal muscular atrophy (SMA) treated with nusinersen, onasemnogene abeparvovec, risdiplam or combination therapies: A systematic review of real-world study data.Eur J Paediatr Neurol. 2022 Jul;39:1-10. doi: 10.1016/j.ejpn.2022.04.006. Epub 2022 Apr 30. Eur J Paediatr Neurol. 2022. PMID: 35533607 Review.
Cited by
-
Clinical perspectives: Treating spinal muscular atrophy.Mol Ther. 2024 Aug 7;32(8):2489-2504. doi: 10.1016/j.ymthe.2024.06.020. Epub 2024 Jun 18. Mol Ther. 2024. PMID: 38894541 Review.
-
Switching from Nusinersen to Risdiplam in Spinal Muscular Atrophy: A Comparative Analysis of Safety, Efficacy, and Economic Impact.Hosp Pharm. 2024 Sep 22:00185787241282213. doi: 10.1177/00185787241282213. Online ahead of print. Hosp Pharm. 2024. PMID: 39544825 Free PMC article. No abstract available.
-
Could choosing risdiplam instead of nusinersen in the treatment of type 1 spinal muscular atrophy be a huge cost-minimization opportunity?Croat Med J. 2024 Oct 31;65(5):454-456. doi: 10.3325/cmj.2024.65.454. Croat Med J. 2024. PMID: 39492456 Free PMC article. No abstract available.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

