A Cellular Potts Model of the interplay of synchronization and aggregation
- PMID: 38435996
- PMCID: PMC10909357
- DOI: 10.7717/peerj.16974
A Cellular Potts Model of the interplay of synchronization and aggregation
Abstract
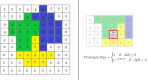
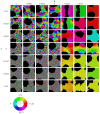
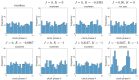
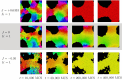
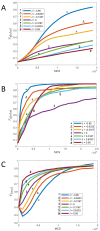
We investigate the behavior of systems of cells with intracellular molecular oscillators ("clocks") where cell-cell adhesion is mediated by differences in clock phase between neighbors. This is motivated by phenomena in developmental biology and in aggregative multicellularity of unicellular organisms. In such systems, aggregation co-occurs with clock synchronization. To account for the effects of spatially extended cells, we use the Cellular Potts Model (CPM), a lattice agent-based model. We find four distinct possible phases: global synchronization, local synchronization, incoherence, and anti-synchronization (checkerboard patterns). We characterize these phases via order parameters. In the case of global synchrony, the speed of synchronization depends on the adhesive effects of the clocks. Synchronization happens fastest when cells in opposite phases adhere the strongest ("opposites attract"). When cells of the same clock phase adhere the strongest ("like attracts like"), synchronization is slower. Surprisingly, the slowest synchronization happens in the diffusive mixing case, where cell-cell adhesion is independent of clock phase. We briefly discuss potential applications of the model, such as pattern formation in the auditory sensory epithelium.
Keywords: Aggregation; Biological clocks; Cellular Potts Model; Mathematical modeling; Synchronization.
©2024 Una and Glimm.
Conflict of interest statement
The authors declare there are no competing interests.
Figures


Similar articles
-
Collective cell movement promotes synchronization of coupled genetic oscillators.Biophys J. 2014 Jul 15;107(2):514-526. doi: 10.1016/j.bpj.2014.06.011. Biophys J. 2014. PMID: 25028893 Free PMC article.
-
Synchronization dynamics in diverse ensemble of noisy phase oscillators with asynchronous phase updates.Phys Rev E Stat Nonlin Soft Matter Phys. 2015 Dec;92(6):062910. doi: 10.1103/PhysRevE.92.062910. Epub 2015 Dec 10. Phys Rev E Stat Nonlin Soft Matter Phys. 2015. PMID: 26764777
-
Random cell movement promotes synchronization of the segmentation clock.Proc Natl Acad Sci U S A. 2010 Mar 16;107(11):4979-84. doi: 10.1073/pnas.0907122107. Epub 2010 Mar 1. Proc Natl Acad Sci U S A. 2010. PMID: 20194769 Free PMC article.
-
What are you synching about? Emerging complexity of Notch signaling in the segmentation clock.Dev Biol. 2020 Apr 1;460(1):40-54. doi: 10.1016/j.ydbio.2019.06.024. Epub 2019 Jul 11. Dev Biol. 2020. PMID: 31302101 Review.
-
Monitoring circadian rhythms of individual cells in plants.J Plant Res. 2018 Jan;131(1):15-21. doi: 10.1007/s10265-017-1001-x. Epub 2017 Dec 4. J Plant Res. 2018. PMID: 29204752 Review.
References
-
- Alber MS, Jiang Y, Kiskowski MA. Lattice gas cellular automation model for rippling and aggregation in myxobacteria. Physica D: Nonlinear Phenomena. 2004;191(3):343–358. doi: 10.1016/j.physd.2003.11.012. - DOI
-
- Barciś A, Bettstetter C. Sandsbots: robots that sync and swarm. IEEE Access. 2020;8:218752–218764. doi: 10.1109/ACCESS.2020.3041393. - DOI
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

