Comparison of outcomes on hypoxia-inducible factor prolyl hydroxylase inhibitors (HIF-PHIs) in anaemia associated with chronic kidney disease: network meta-analyses in dialysis and non-dialysis dependent populations
- PMID: 38250252
- PMCID: PMC10799328
- DOI: 10.1093/ckj/sfad298
Comparison of outcomes on hypoxia-inducible factor prolyl hydroxylase inhibitors (HIF-PHIs) in anaemia associated with chronic kidney disease: network meta-analyses in dialysis and non-dialysis dependent populations
Abstract
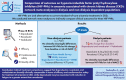
Background: Hypoxia-inducible factor prolyl hydroxylase inhibitors (HIF-PHIs) are oral alternatives to current standard-of-care treatments for anaemia in chronic kidney disease (CKD). We conducted network meta-analyses to indirectly compare clinical outcomes for three HIF-PHIs in dialysis and non-dialysis populations with anaemia in CKD.
Methods: The evidence base comprised phase III, randomised, controlled trials evaluating daprodustat, roxadustat, or vadadustat. Three outcomes were evaluated: efficacy [change from baseline in haemoglobin (Hgb)], cardiovascular safety [time to first major adverse cardiovascular event (MACE)] and quality of life [change from baseline in 36-Item Short Form Health Survey (SF-36) Vitality score]. Analyses were performed separately for all patients and for erythropoiesis-stimulating agent (ESA) non-users at baseline (non-dialysis population) or prevalent dialysis patients (dialysis population). Bayesian Markov Chain Monte Carlo methods with non-informative priors were used to estimate the posterior probability distribution and generate pairwise treatment comparisons. Point estimates (medians of posterior distributions) and 95% credible intervals (CrI) were calculated.
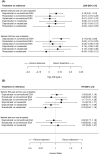
Results: Seventeen trials were included. In non-dialysis patients, there were no clinically meaningful differences between the three HIF-PHIs with respect to Hgb change from baseline [all patients analysis (total n = 7907): daprodustat vs. roxadustat, 0.09 g/dL (95% CrI -0.14, 0.31); daprodustat vs. vadadustat, 0.09 g/dL (-0.04, 0.21); roxadustat vs. vadadustat, 0.00 g/dL (-0.22, 0.22)] or risk of MACE [all patients analysis (total n = 7959): daprodustat vs. roxadustat, hazard ratio (HR) 1.16 (95% CrI 0.76, 1.77); daprodustat vs. vadadustat, 0.88 (0.71, 1.09); roxadustat vs. vadadustat, 0.76 (0.50, 1.16)]. Daprodustat showed a greater increase in SF-36 Vitality compared with roxadustat [total n = 4880; treatment difference 4.70 points (95% CrI 0.08, 9.31)]. In dialysis patients, Hgb change from baseline was higher with daprodustat and roxadustat compared with vadadustat [all patients analysis (total n = 11 124): daprodustat, 0.34 g/dL (0.22, 0.45); roxadustat, 0.38 g/dL (0.27, 0.49)], while there were no clinically meaningful differences in the risk of MACE between the HIF-PHIs [all patients analysis (total n = 12 320): daprodustat vs. roxadustat, HR 0.89 (0.73, 1.08); daprodustat vs. vadadustat, HR 0.99 (0.82, 1.21); roxadustat vs. vadadustat, HR 1.12 (0.92, 1.37)]. Results were similar in analyses of ESA non-users and prevalent dialysis patients.
Conclusions: In the setting of anaemia in CKD, indirect treatment comparisons suggest that daprodustat, roxadustat, and vadadustat are broadly clinically comparable in terms of efficacy and cardiovascular safety (precision was low for the latter), while daprodustat may be associated with reduction in fatigue to a greater extent than roxadustat.
Keywords: anaemia; chronic kidney disease; hypoxia-inducible factor prolyl hydroxylase inhibitors; network meta-analysis; outcomes.
© The Author(s) 2023. Published by Oxford University Press on behalf of the ERA.
Conflict of interest statement
A.S. is an employee of GSK. R.D.L. has received research support from Bristol Myers Squibb, GSK, Medtronic, and Pfizer; and consulting fees from Bayer, Boehringer Ingelheim, Bristol Myers Squibb, Daiichi-Sankyo, GSK, Medtronic, Merck, Pfizer, and Portola. C.P.K. has received consulting fees from Abbott, Akebia, AstraZeneca, Bayer, Boehringer Ingelheim, Cara Therapeutics, CSL Behring, CSL Vifor, GSK, Pharmacosmos, ProKidney, Rockwell, Takeda, and Tricida, outside of the submitted work. A.C. has received research grants from CSL Vifor; consultancy fees from Astellas, AstraZeneca, Boehringer Ingelheim, CSL Vifor, GSK, Novo Nordisk, and Otsuka; speaker fees from Amgen, Astellas, AstraZeneca, Bayer, CSL Vifor, GSK, Novo Nordisk, and Sanofi (Mexico) and travel support from Astellas, AstraZeneca, and GSK, outside the submitted work. AC is also a member of the Catalan Registry of Renal Patients (RMRC). S.A.M was an employee of GSK at the time of the study. N.B. is an employee of, and shareholder in, GSK. T.J.K. is an employee of, and shareholder in, GSK. V.G.-H. is an employee of Analysis Group, Inc., which received funding from GSK to conduct this research. R.A. is an employee of Analysis Group, Inc., which received funding from GSK to conduct this research. R.R.C. is an employee of and shareholder in GSK. K.L.J. has received consulting fees from GSK, Vifor, and Akebia. A.J.S. has received consultancy fees from Analysis Group, Inc. I.D. has received research grants from Sanofi, NIHR Clinical Research Network (West Midlands, UK), and Kidney Research UK; and honoraria for advisory boards and speaker meetings from GSK, AstraZeneca, Sanofi, and Vifor.
Figures



Similar articles
-
A network meta-analysis of the efficacy of hypoxia-inducible factor prolyl-hydroxylase inhibitors in dialysis chronic kidney disease.Aging (Albany NY). 2023 Mar 27;15(6):2237-2274. doi: 10.18632/aging.204611. Epub 2023 Mar 27. Aging (Albany NY). 2023. PMID: 36988549 Free PMC article.
-
Safety and Efficacy of Hypoxia-Inducible Factor-Prolyl Hydroxylase Inhibitors vs. Erythropoietin-Stimulating Agents in Treating Anemia in Renal Patients (With or Without Dialysis): A Meta-Analysis and Systematic Review.Cureus. 2023 Oct 21;15(10):e47430. doi: 10.7759/cureus.47430. eCollection 2023 Oct. Cureus. 2023. PMID: 38021836 Free PMC article. Review.
-
The Comparison between Vadadustat and Daprodustat Regarding Dose, Cost, and Safety of Treatment for Renal Anemia in Non-dialysis Patients with Chronic Kidney Diseases.Intern Med. 2024 Jul 1;63(13):1855-1861. doi: 10.2169/internalmedicine.2501-23. Epub 2023 Nov 6. Intern Med. 2024. PMID: 37926547 Free PMC article.
-
Safety of HIF prolyl hydroxylase inhibitors for anemia in dialysis patients: a systematic review and network meta-analysis.Front Pharmacol. 2023 May 24;14:1163908. doi: 10.3389/fphar.2023.1163908. eCollection 2023. Front Pharmacol. 2023. PMID: 37292157 Free PMC article.
-
Efficacy and safety of HIF prolyl-hydroxylase inhibitor vs epoetin and darbepoetin for anemia in chronic kidney disease patients not undergoing dialysis: A network meta-analysis.Pharmacol Res. 2020 Sep;159:105020. doi: 10.1016/j.phrs.2020.105020. Epub 2020 Jun 16. Pharmacol Res. 2020. PMID: 32561478 Review.
Cited by
-
Chemistry, Analysis, and Biological Aspects of Daprodustat, A New Hypoxia Inducible Factor Prolyl Hydroxylase Inhibitor: A Comprehensive Review.Mini Rev Med Chem. 2024;24(20):1847-1855. doi: 10.2174/0113895575293447240424052516. Mini Rev Med Chem. 2024. PMID: 38685804 Review.
-
Hypoxia-Inducible Factor Prolyl Hydroxylase Inhibitors as a New Treatment Option for Anemia in Chronic Kidney Disease.Biomedicines. 2024 Aug 18;12(8):1884. doi: 10.3390/biomedicines12081884. Biomedicines. 2024. PMID: 39200348 Free PMC article. Review.
References
-
- Kovesdy CP, Davis JR, Duling Iet al. . Prevalence of anaemia in adults with chronic kidney disease in a representative sample of the United States population: analysis of the 1999–2018 National Health and Nutrition Examination Survey. Clin Kidney J 2023;16:303–11. 10.1093/ckj/sfac240 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

