Development of nanoparticles based on amphiphilic cyclodextrins for the delivery of active substances
- PMID: 38110013
- PMCID: PMC11641101
- DOI: 10.1016/j.ijpharm.2023.123723
Development of nanoparticles based on amphiphilic cyclodextrins for the delivery of active substances
Abstract
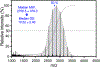
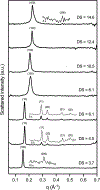
Although amphiphilic cyclodextrin derivatives (ACDs) serve as valuable building blocks for nanomedicine formulations, their widespread production still encounters various challenges, limiting large-scale manufacturing. This work focuses on a robust alternative pathway using mineral base catalysis to transesterify β-cyclodextrin with long-chain vinyl esters, yielding ACD with modular and controlled hydrocarbon chain grafting. ACDs with a wide range of degrees of substitution (DS) were reliably synthesized, as indicated by extensive physicochemical characterization, including MALDI-TOF mass spectrometry. The influence of various factors, including the type of catalyst and the length of the hydrocarbon moiety of the vinyl ester, was studied in detail. ACDs were assessed for their ability to form colloidal suspensions by nanoprecipitation, with or without PEGylated phospholipid. Small-angle X-ray scattering and cryo-electron microscopy revealed the formation of nanoparticles with distinct ultrastructures depending on the DS: an onion-like structure for low and very high DS, and reversed hexagonal organization for DS between 4.5 and 6.1. We confirmed the furtivity of the PEGylated versions of the nanoparticles through complement activation experiments and that they were well tolerated in-vivo on a zebrafish larvae model after intravenous injection. Furthermore, a biodistribution experiment showed that the nanoparticles left the bloodstream within 10 h after injection and were phagocytosed by macrophages.
Keywords: Amphiphilic cyclodextrin; Complement activation; Nanoparticles; PEGylation; Zebrafish larvae.
Copyright © 2023 Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


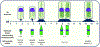


Similar articles
-
Ceftazidime with avibactam for treating severe aerobic Gram-negative bacterial infections: technology evaluation to inform a novel subscription-style payment model.Health Technol Assess. 2024 Oct;28(73):1-230. doi: 10.3310/YAPL9347. Health Technol Assess. 2024. PMID: 39487661 Free PMC article.
-
Defining the optimum strategy for identifying adults and children with coeliac disease: systematic review and economic modelling.Health Technol Assess. 2022 Oct;26(44):1-310. doi: 10.3310/ZUCE8371. Health Technol Assess. 2022. PMID: 36321689 Free PMC article.
-
Depressing time: Waiting, melancholia, and the psychoanalytic practice of care.In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. PMID: 36137063 Free Books & Documents. Review.
-
Falls prevention interventions for community-dwelling older adults: systematic review and meta-analysis of benefits, harms, and patient values and preferences.Syst Rev. 2024 Nov 26;13(1):289. doi: 10.1186/s13643-024-02681-3. Syst Rev. 2024. PMID: 39593159 Free PMC article.
-
Couple therapy for depression.Cochrane Database Syst Rev. 2018 Jun 8;6(6):CD004188. doi: 10.1002/14651858.CD004188.pub3. Cochrane Database Syst Rev. 2018. PMID: 29882960 Free PMC article. Review.
Cited by
-
The Many Faces of Cyclodextrins within Self-Assembling Polymer Nanovehicles: From Inclusion Complexes to Valuable Structural and Functional Elements.Int J Mol Sci. 2024 Sep 1;25(17):9516. doi: 10.3390/ijms25179516. Int J Mol Sci. 2024. PMID: 39273469 Free PMC article. Review.
-
Monomeric, Oligomeric, Polymeric, and Supramolecular Cyclodextrins as Catalysts for Green Chemistry.Research (Wash D C). 2024 Sep 9;7:0466. doi: 10.34133/research.0466. eCollection 2024. Research (Wash D C). 2024. PMID: 39253101 Free PMC article.
References
-
- Addgene URL https://www.addgene.org/58935/ (accessed 4.17.23).
-
- Aytac Z, Uyar T, 2016. Antioxidant activity and photostability of α-tocopherol/β-cyclodextrin inclusion complex encapsulated electrospun polycaprolactone nanofibers. Eur. Polym. J 79, 140–149. 10.1016/j.eurpolymj.2016.04.029 - DOI
-
- Brady B, Lynam N, O’Sullivan T, Ahern C, Darcy R, 2000. 6A-O-p-Toluenesulfonyl-β-cyclodextrin. Org. Synth 77, 220–221. 10.15227/orgsyn.077.0220 - DOI
-
- Bandi SP, Kumbhar YS, Venuganti VVK, 2020. Effect of particle size and surface charge of nanoparticles in penetration through intestinal mucus barrier. J. Nanoparticle Res 22, 62. 10.1007/s11051-020-04785-y - DOI
-
- Benito JM, García Fernández JM, 2019. Protecting Groups: Strategies and Applications in Carbohydrate Chemistry, 1st ed. Wiley. 10.1002/9783527697014 - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

