A systematic review of passing fit testing of the masks and respirators used during the COVID-19 pandemic: Part 1-quantitative fit test procedures
- PMID: 37883443
- PMCID: PMC10602271
- DOI: 10.1371/journal.pone.0293129
A systematic review of passing fit testing of the masks and respirators used during the COVID-19 pandemic: Part 1-quantitative fit test procedures
Abstract
Background: During respiratory infection pandemics, masks and respirators are highly sought after, especially for frontline healthcare workers and patients carrying respiratory viruses. The objective of this study was to systematically review fit test pass rates and identify factors influencing the fitting characteristics.
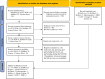
Methods: Potentially relevant studies were identified using PubMed, Scopus, Web of Science, and Science Direct during the COVID-19 pandemic from February 5, 2020, to March 21, 2023. The search strategy using the following keywords was conducted: Quantitative Fit Test, Condensation Nuclei Counter, Controlled Negative Pressure, PortaCount, Sibata, Accufit, Fit, Seal, Mask, Respirator, Respiratory Protective Device, Respiratory Protective Equipment, Protective Device, Personal Protective Equipment, COVID-19, Coronavirus, and SARS-CoV-2. The quality of the included studies was also assessed using the Newcastle-Ottawa scale.
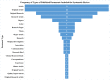
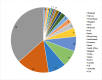
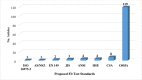
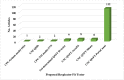
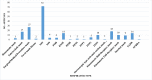
Results: A total of 137 articles met the eligibility criteria. Fifty articles had a quality score of less than 7 (good quality). A total of 21 studies had a fit test pass rate of less than 50%. 26 studies on disposable respirators and 11 studies on reusable respirators had an FF of less than 50 and less than 200, respectively. The most influential factors include respirator brand/model, style, gender, ethnicity, facial dimensions, facial hair, age, reuse, extensive movement, seal check, comfort and usability assessment, and training.
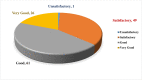
Conclusion: 37.36% of the disposable respirator studies and 43% of the reusable respirator studies did not report fit test results. 67.86% of the disposable respirator studies had a fit test pass rate greater than 50%, and 35.84% of these studies had an FF greater than 100. Also, 85.71% of the reusable respirator studies had a fit test pass rate greater than 50%, and 52.77% of these studies had an FF greater than 1000. Overall, the fit test pass rate was relatively acceptable. Newly developed or modified respirators must undergo reliable testing to ensure the protection of HCWs. Subject and respirator characteristics should be considered when implementing fit testing protocols. An optimal fit test panel should be developed prior to respirator design, certification, procurement decisions, and selection procedures.
Copyright: © 2023 Fakherpour et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Similar articles
-
Development, manufacturing, and preliminary validation of a reusable half-face respirator during the COVID-19 pandemic.PLoS One. 2021 Mar 17;16(3):e0247575. doi: 10.1371/journal.pone.0247575. eCollection 2021. PLoS One. 2021. PMID: 33730106 Free PMC article.
-
Subject validation of reusable N95 stop-gap filtering facepiece respirators in COVID-19 pandemic.PLoS One. 2020 Nov 13;15(11):e0242304. doi: 10.1371/journal.pone.0242304. eCollection 2020. PLoS One. 2020. PMID: 33186406 Free PMC article.
-
Under-mask beard covers achieve an adequate seal with tight-fitting disposable respirators using quantitative fit testing.J Hosp Infect. 2022 Oct;128:8-12. doi: 10.1016/j.jhin.2022.05.015. Epub 2022 May 31. J Hosp Infect. 2022. PMID: 35662553
-
Overview of tight fit and infection prevention benefits of respirators (filtering face pieces).J Hosp Infect. 2023 Apr;134:89-96. doi: 10.1016/j.jhin.2023.01.009. Epub 2023 Feb 3. J Hosp Infect. 2023. PMID: 36738992 Free PMC article. Review.
-
The role of fit testing N95/FFP2/FFP3 masks: a narrative review.Anaesthesia. 2021 Jan;76(1):91-100. doi: 10.1111/anae.15261. Epub 2020 Sep 15. Anaesthesia. 2021. PMID: 32932556 Review.
Cited by
-
Masks and respirators for prevention of respiratory infections: a state of the science review.Clin Microbiol Rev. 2024 Jun 13;37(2):e0012423. doi: 10.1128/cmr.00124-23. Epub 2024 May 22. Clin Microbiol Rev. 2024. PMID: 38775460 Review.
References
-
- NIOSH. Hierarchy of Controls. 2023 Jun 17 [cited 2023 July 14]. In: CDC Web site [Internet]. Available from: https://www.cdc.gov/niosh/topics/hierarchy/default.html.
-
- OECD. The face mask global value chain in the COVID-19 outbreak: Evidence and policy lessons,OECD Policy Responses to Coronavirus (COVID-19). OECD Publishing, Paris; 2020.
-
- Regli A, Thalayasingam P, Bell E, Sommerfield A, von Ungern-Sternberg BS. More than half of front-line healthcare workers unknowingly used an N95/P2 mask without adequate airborne protection: An audit in a tertiary institution. Anaesth Intensive Care. 2021;49(5):404–11. doi: 10.1177/0310057X211007861 - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

