Tafasitamab and lenalidomide in large B-cell lymphoma: real-world outcomes in a multicenter retrospective study
- PMID: 37738563
- PMCID: PMC10797539
- DOI: 10.1182/blood.2023021274
Tafasitamab and lenalidomide in large B-cell lymphoma: real-world outcomes in a multicenter retrospective study
Abstract
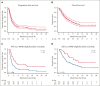
In this real-world evaluation of tafasitamab-lenalidomide (TL) in relapsed or refractory LBCL, patients receiving TL had higher rates of comorbidities and high-risk disease characteristics, and substantially lower progression-free survival and overall survival, compared with the L-MIND registration clinical trial for TL.
© 2023 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: P.F.C. has received research funding from ADC Therapeutics, Genentech, and AbbVie and honoraria for participation in advisory boards/consulting from ADC Therapeutics, Beigene, BMS, Genentech, Kite, Lilly, MEI Pharma, Novartis, and Takeda. D.A.B. has received research funding from Incyte/Morphosys, Novartis, and Nurix Therapeutics and honoraria for consulting from SeaGen, Kite/Gilead, Nurix Therapeutics, and Novartis. L.L. has received honoraria for consulting and/or participation in speaker bureau from Kite/Gilead, Beigene, Pharmacyclics, AbbVie, Genmab, SeaGen, Janssen, AstraZeneca, Eli Lilly, Epizyme, TG Therapeutics, Merck, and ADC Therapeutics. L.J.N. has received honoraria for participation in advisory boards/consulting from AbbVie, ADC Therapeutics, Atara Biotherapeutics, BMS/Celgene, Caribou Biosciences, Daiichi Sankyo, Epizyme, Genentech/Roche, Genmab, Janssen, Incyte, Morphosys, Novartis, and Takeda and research support from BMS/Celgene, Caribou Biosciences, Daiichi Sankyo, Epizyme, Genentech/Roche, Genmab, Gilead/Kite, IGM Biosciences, Janssen, Novartis, and Takeda. J.L.C. has received research support (to institution) from Genentech, Merck, Bayer, and AbbVie and honoraria for consulting from ADC Therapeutics, Kite Pharma, Genmab, and Incyte/MorphoSys. J.S.A. has received honoraria for consulting from AbbVie, AstraZeneca, BeiGene, BMS, Caribou Biosciences, Cellectar, Century Therapeutics, Epizyme, Genentech, Genmab, Incyte, Interius, Janssen, Kite Pharma, Kymera, Lilly, MorphoSys, Mustang Bio, Ono Pharma, Regeneron, and Takeda and research support (to institution) from BMS, Cellectis, Merck, Mustang Bio, and Seattle Genetics. G.S.N. has received honoraria for consultancy from AbbVie, ADC Therapeutics, Bantam Pharmaceutical LLC, Blueprint Medicines, Bristol Myers Squibb, Celgene Corporation, Curis, Debiopharm, F. Hoffmann-La Roche Limited, Fate Therapeutics, Genentech, Incyte, Karyopharm Therapeutics, Kite Pharma, Kymera Therapeutics, MEI Pharma, MorphoSys AG, Ryvu Therapeutics, Seagen, Selvita Inc, TG Therapeutics, and Zai Lab Limited and research funding from Bristol Myers Squibb and MorphoSys and is a member on a board of directors or advisory committee for Karyopharm Therapeutics, Ryvu Therapeutics, and Fate Therapeutics. K.M. has received research funding from Pharmacyclics, Novartis, Merck, BMS, and Celgene and honoraria for consulting from AbbVie, ADC Therapeutics, AstraZeneca, BMS, Celgene, Gilead/Kite, GenMab, Genentech, Incyte, Janssen, Merck, Morphosys, Lilly, and Pharmacyclics. S.C.R. has received research funding from Constellation, Karyopharm, and Genentech/Roche and honoraria for consulting from ADC, Genmab, Karyopharm, Kite, and Seagen. B.K. has received honoraria for consulting from AbbVie, AstraZeneca, ADCT, BeiGene, BMS, Genentech, Genmab, Gilead, Janssen, and Lilly and research funding from Genentech, ADCT, AstraZeneca, and BeiGene. C.L.B. is currently employed at Roche/Genentech; participation in the study occurred prior to employment at Roche/Genentech. G.S. has received, in the last 12 months, financial compensation for participating in advisory boards or consulting from AbbVie, ATB Therapeutics, Beigene, BMS/Celgene, Debiopharm, Genentech/Roche, Genmab, Incyte, Ipsen, Janssen, Kite/Gilead, Loxo/Lilly, Merck, Molecular Partners, Nordic Nanovector, Novartis, Nurix, and Orna; is shareholder of Owkin; and has received research support managed by his institution from Genentech, Janssen and Ipsen. The remaining authors declare no competing financial interests.
The current affiliation for C.L.B. is Roche/Genentech, San Francisco, CA.
Figures
Comment in
-
Tribulations of trials in aggressive lymphoma.Blood. 2023 Dec 28;142(26):2232-2234. doi: 10.1182/blood.2023022382. Blood. 2023. PMID: 38153771 No abstract available.
Similar articles
-
Safety and activity of lenalidomide in combination with obinutuzumab in patients with relapsed indolent non-Hodgkin lymphoma: a single group, open-label, phase 1/2 trial.EClinicalMedicine. 2024 Jul 27;74:102747. doi: 10.1016/j.eclinm.2024.102747. eCollection 2024 Aug. EClinicalMedicine. 2024. PMID: 39161543 Free PMC article.
-
A Phase II Trial of Bortezomib and Vorinostat in Mantle Cell Lymphoma and Diffuse Large B-cell Lymphoma.Clin Lymphoma Myeloma Leuk. 2018 Sep;18(9):569-575.e1. doi: 10.1016/j.clml.2018.05.023. Epub 2018 Jun 6. Clin Lymphoma Myeloma Leuk. 2018. PMID: 30122201 Free PMC article. Clinical Trial.
-
Autologous stem cell transplant in fit patients with refractory or early relapsed diffuse large B-cell lymphoma that responded to salvage chemotherapy.Haematologica. 2024 Jul 1;109(7):2186-2195. doi: 10.3324/haematol.2023.284704. Haematologica. 2024. PMID: 38235513 Free PMC article.
-
Maintenance therapy for chronic lymphocytic leukaemia.Cochrane Database Syst Rev. 2024 Jan 4;1(1):CD013474. doi: 10.1002/14651858.CD013474.pub2. Cochrane Database Syst Rev. 2024. PMID: 38174814 Free PMC article. Review.
-
Daratumumab and antineoplastic therapy versus antineoplastic therapy only for adults with newly diagnosed multiple myeloma ineligible for transplant.Cochrane Database Syst Rev. 2024 May 2;5(5):CD013595. doi: 10.1002/14651858.CD013595.pub2. Cochrane Database Syst Rev. 2024. PMID: 38695605 Review.
Cited by
-
Treatment of Relapsed or Refractory Diffuse Large B-Cell Lymphoma: New Approved Options.J Clin Med. 2023 Dec 22;13(1):70. doi: 10.3390/jcm13010070. J Clin Med. 2023. PMID: 38202077 Free PMC article. Review.
-
Tumor Biology Hides Novel Therapeutic Approaches to Diffuse Large B-Cell Lymphoma: A Narrative Review.Int J Mol Sci. 2024 Oct 23;25(21):11384. doi: 10.3390/ijms252111384. Int J Mol Sci. 2024. PMID: 39518937 Free PMC article. Review.
-
The rules of T-cell engagement: current state of CAR T cells and bispecific antibodies in B-cell lymphomas.Blood Adv. 2024 Sep 10;8(17):4700-4710. doi: 10.1182/bloodadvances.2021004535. Blood Adv. 2024. PMID: 39042891 Free PMC article. Review.
-
Novel Targets and Advanced Therapies in Diffuse Large B Cell Lymphomas.Cancers (Basel). 2024 Jun 17;16(12):2243. doi: 10.3390/cancers16122243. Cancers (Basel). 2024. PMID: 38927948 Free PMC article. Review.
-
Tafasitamab for the treatment of patients with diffuse large B-cell lymphoma.Hum Vaccin Immunother. 2024 Dec 31;20(1):2309701. doi: 10.1080/21645515.2024.2309701. Epub 2024 Feb 1. Hum Vaccin Immunother. 2024. PMID: 38299612 Free PMC article.
References
-
- US Food and Drug Administration FDA grants accelerated approval to tafasitamab-cxix for diffuse large B-cell lymphoma. Last updated 3 August 2020. https://www.fda.gov/drugs/drug-approvals-and-databases/fda-grants-accele...
-
- Salles G, Duell J, González Barca E, et al. Tafasitamab plus lenalidomide in relapsed or refractory diffuse large B-cell lymphoma (L-MIND): a multicentre, prospective, single-arm, phase 2 study. Lancet Oncol. 2020;21(7):978–988. - PubMed
-
- Cheson BD, Ansell S, Schwartz L, et al. Refinement of the Lugano Classification lymphoma response criteria in the era of immunomodulatory therapy. Blood. 2016;128(21):2489–2496. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources