The bridge between cell survival and cell death: reactive oxygen species-mediated cellular stress
- PMID: 37534225
- PMCID: PMC10390897
- DOI: 10.17179/excli2023-6221
The bridge between cell survival and cell death: reactive oxygen species-mediated cellular stress
Abstract
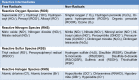
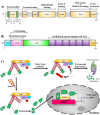
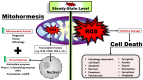
As a requirement of aerobic metabolism, regulation of redox homeostasis is indispensable for the continuity of living homeostasis and life. Since the stability of the redox state is necessary for the maintenance of the biological functions of the cells, the balance between the pro-oxidants, especially ROS and the antioxidant capacity is kept in balance in the cells through antioxidant defense systems. The pleiotropic transcription factor, Nrf2, is the master regulator of the antioxidant defense system. Disruption of redox homeostasis leads to oxidative and reductive stress, bringing about multiple pathophysiological conditions. Oxidative stress characterized by high ROS levels causes oxidative damage to biomolecules and cell death, while reductive stress characterized by low ROS levels disrupt physiological cell functions. The fact that ROS, which were initially attributed as harmful products of aerobic metabolism, at the same time function as signal molecules at non-toxic levels and play a role in the adaptive response called mithormesis points out that ROS have a dose-dependent effect on cell fate determination. See also Figure 1(Fig. 1).
Keywords: Nrf2; antioxidant defense systems; cell death pathways; mitohormesis; oxidative and reductive stress; redox homeostasis.
Copyright © 2023 Vardar Acar et al.
Figures





Similar articles
-
Regulation of the Nrf2 antioxidant pathway by microRNAs: New players in micromanaging redox homeostasis.Free Radic Biol Med. 2013 Sep;64:4-11. doi: 10.1016/j.freeradbiomed.2013.07.025. Epub 2013 Jul 21. Free Radic Biol Med. 2013. PMID: 23880293 Review.
-
Oxidative stress and protein aggregation during biological aging.Exp Gerontol. 2001 Sep;36(9):1539-50. doi: 10.1016/s0531-5565(01)00139-5. Exp Gerontol. 2001. PMID: 11525876 Review.
-
Transcriptional activation of antioxidant gene expression by Nrf2 protects against mitochondrial dysfunction and neuronal death associated with acute and chronic neurodegeneration.Exp Neurol. 2020 Jun;328:113247. doi: 10.1016/j.expneurol.2020.113247. Epub 2020 Feb 12. Exp Neurol. 2020. PMID: 32061629 Free PMC article. Review.
-
Redox and oxidant-mediated regulation of apoptosis signaling pathways: immuno-pharmaco-redox conception of oxidative siege versus cell death commitment.Int Immunopharmacol. 2004 Apr;4(4):475-93. doi: 10.1016/j.intimp.2004.02.002. Int Immunopharmacol. 2004. PMID: 15099526 Review.
-
Responses to reductive stress in the cardiovascular system.Free Radic Biol Med. 2017 Aug;109:114-124. doi: 10.1016/j.freeradbiomed.2016.12.006. Epub 2016 Dec 8. Free Radic Biol Med. 2017. PMID: 27940350 Free PMC article. Review.
Cited by
-
The role of reactive oxygen species in severe acute respiratory syndrome coronavirus 2 (SARS-COV-2) infection-induced cell death.Cell Mol Biol Lett. 2024 Nov 8;29(1):138. doi: 10.1186/s11658-024-00659-6. Cell Mol Biol Lett. 2024. PMID: 39516736 Free PMC article. Review.
-
Application of Nanomaterials and Related Drug Delivery Systems in Autophagy.Molecules. 2024 Jul 26;29(15):3513. doi: 10.3390/molecules29153513. Molecules. 2024. PMID: 39124918 Free PMC article. Review.
References
-
- Al-Sawaf O, Clarner T, Fragoulis A, Kan YW, Pufe T, Streetz K, et al. Nrf2 in health and disease: current and future clinical implications. Clin Sci. 2015;129:989–999. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
