Single-cell phenotypic plasticity modulates social behavior in Dictyostelium discoideum
- PMID: 37235054
- PMCID: PMC10206496
- DOI: 10.1016/j.isci.2023.106783
Single-cell phenotypic plasticity modulates social behavior in Dictyostelium discoideum
Abstract
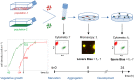
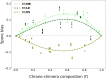
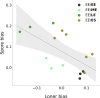
In Dictyostelium chimeras, "cheaters" are strains that positively bias their contribution to the pool of spores, i.e., the reproductive cells resulting from development. On evolutionary time scales, the selective advantage; thus, gained by cheaters is predicted to undermine collective functions whenever social behaviors are genetically determined. Genotypes; however, are not the sole determinant of spore bias, but the relative role of genetic and plastic differences in evolutionary success is unclear. Here, we study chimeras composed of cells harvested in different phases of population growth. We show that such heterogeneity induces frequency-dependent, plastic variation in spore bias. In genetic chimeras, the magnitude of such variation is not negligible and can even reverse the classification of a strain's social behavior. Our results suggest that differential cell mechanical properties can underpin, through biases emerging during aggregation, a "lottery" in strains' reproductive success that may counter the evolution of cheating.
Keywords: Cell biology; Evolutionary biology; Microbiology.
© 2023 The Authors.
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
In the social amoeba Dictyostelium discoideum, shortened stalks may limit obligate cheater success even when exploitable partners are available.PeerJ. 2024 Mar 29;12:e17118. doi: 10.7717/peerj.17118. eCollection 2024. PeerJ. 2024. PMID: 38562996 Free PMC article.
-
Quantification of social behavior in D. discoideum reveals complex fixed and facultative strategies.Curr Biol. 2009 Aug 25;19(16):1373-7. doi: 10.1016/j.cub.2009.06.058. Epub 2009 Jul 23. Curr Biol. 2009. PMID: 19631539
-
Evolving social behavior through selection of single-cell adhesion in Dictyostelium discoideum.iScience. 2022 Aug 25;25(9):105006. doi: 10.1016/j.isci.2022.105006. eCollection 2022 Sep 16. iScience. 2022. PMID: 36105585 Free PMC article.
-
Evolution of cooperation and control of cheating in a social microbe.Proc Natl Acad Sci U S A. 2011 Jun 28;108 Suppl 2(Suppl 2):10855-62. doi: 10.1073/pnas.1102451108. Epub 2011 Jun 20. Proc Natl Acad Sci U S A. 2011. PMID: 21690338 Free PMC article. Review.
-
Cooperation and conflict in the social amoeba Dictyostelium discoideum.Int J Dev Biol. 2019;63(8-9-10):371-382. doi: 10.1387/ijdb.190158jm. Int J Dev Biol. 2019. PMID: 31840776 Review.
References
-
- Forget M., Adiba S., De Monte S. Social conflicts in Dictyostelium discoideum : a matter of scales. Peer Community Journal. 2021;1:e58. doi: 10.24072/pcjournal.39. - DOI
-
- Raper K.B. Pseudoplasmodium formation and organization in Dictyostelium discoideum. J. Elisha Mitchell Sci. Soc. 1940;56:241–282.
LinkOut - more resources
Full Text Sources

