Vitamin C Regulates the Profibrotic Activity of Fibroblasts in In Vitro Replica Settings of Myocardial Infarction
- PMID: 37176085
- PMCID: PMC10179686
- DOI: 10.3390/ijms24098379
Vitamin C Regulates the Profibrotic Activity of Fibroblasts in In Vitro Replica Settings of Myocardial Infarction
Abstract
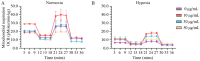
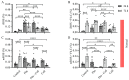
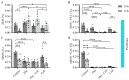
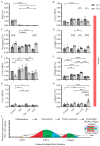
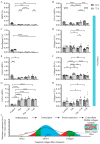
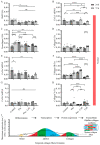
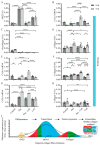
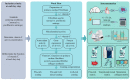
Extracellular collagen remodeling is one of the central mechanisms responsible for the structural and compositional coherence of myocardium in patients undergoing myocardial infarction (MI). Activated primary cardiac fibroblasts following myocardial infarction are extensively investigated to establish anti-fibrotic therapies to improve left ventricular remodeling. To systematically assess vitamin C functions as a potential modulator involved in collagen fibrillogenesis in an in vitro model mimicking heart tissue healing after MI. Mouse primary cardiac fibroblasts were isolated from wild-type C57BL/6 mice and cultured under normal and profibrotic (hypoxic + transforming growth factor beta 1) conditions on freshly prepared coatings mimicking extracellular matrix (ECM) remodeling during healing after an MI. At 10 μg/mL, vitamin C reprogramed the respiratory mitochondrial metabolism, which is effectively associated with a more increased accumulation of intracellular reactive oxygen species (iROS) than the number of those generated by mitochondrial reactive oxygen species (mROS). The mRNA/protein expression of subtypes I, III collagen, and fibroblasts differentiations markers were upregulated over time, particularly in the presence of vitamin C. The collagen substrate potentiated the modulator role of vitamin C in reinforcing the structure of types I and III collagen synthesis by reducing collagen V expression in a timely manner, which is important in the initiation of fibrillogenesis. Altogether, our study evidenced the synergistic function of vitamin C at an optimum dose on maintaining the equilibrium functionality of radical scavenger and gene transcription, which are important in the initial phases after healing after an MI, while modulating the synthesis of de novo collagen fibrils, which is important in the final stage of tissue healing.
Keywords: antioxidant capacity; collagen modulator; fibroblast; gene transcription regulator; vitamin C.
Conflict of interest statement
The authors declare that the manuscript was prepared in the absence of any conflict of interest.
Figures
Similar articles
-
Osteoglycin prevents cardiac dilatation and dysfunction after myocardial infarction through infarct collagen strengthening.Circ Res. 2015 Jan 30;116(3):425-36. doi: 10.1161/CIRCRESAHA.116.304599. Epub 2014 Dec 17. Circ Res. 2015. PMID: 25520363
-
Ectonucleoside triphosphate diphosphohydrolase-1 (CD39) impacts TGF-β1 responses: insights into cardiac fibrosis and function following myocardial infarction.Am J Physiol Heart Circ Physiol. 2022 Dec 1;323(6):H1244-H1261. doi: 10.1152/ajpheart.00138.2022. Epub 2022 Oct 14. Am J Physiol Heart Circ Physiol. 2022. PMID: 36240436 Free PMC article.
-
Essential role of Smad3 in infarct healing and in the pathogenesis of cardiac remodeling.Circulation. 2007 Nov 6;116(19):2127-38. doi: 10.1161/CIRCULATIONAHA.107.704197. Epub 2007 Oct 22. Circulation. 2007. PMID: 17967775
-
Regulation and role of myocardial collagen matrix remodeling in hypertensive heart disease.Adv Exp Med Biol. 1997;432:35-44. doi: 10.1007/978-1-4615-5385-4_4. Adv Exp Med Biol. 1997. PMID: 9433509 Review.
-
Role of angiotensin II and prostaglandin E2 in regulating cardiac fibroblast collagen turnover.Am J Cardiol. 1995 Nov 2;76(13):8D-13D. doi: 10.1016/s0002-9149(99)80485-8. Am J Cardiol. 1995. PMID: 7495221 Review.
Cited by
-
Vitamin C as Scavenger of Reactive Oxygen Species during Healing after Myocardial Infarction.Int J Mol Sci. 2024 Mar 7;25(6):3114. doi: 10.3390/ijms25063114. Int J Mol Sci. 2024. PMID: 38542087 Free PMC article. Review.
-
From Cardiovascular Prevention and Differential Diagnosis to the Treatment of Myocardial Damage-Experimental and Human Perspectives.Int J Mol Sci. 2023 Nov 30;24(23):16997. doi: 10.3390/ijms242316997. Int J Mol Sci. 2023. PMID: 38069315 Free PMC article.
-
Vegetable and Fruit Intake Variety and Cardiovascular Health and Mortality: A Systematic Review and Meta-Analysis of Observational Studies.Nutrients. 2023 Nov 24;15(23):4913. doi: 10.3390/nu15234913. Nutrients. 2023. PMID: 38068771 Free PMC article. Review.
References
-
- Virani S.S., Alonso A., Benjamin E.J., Bittencourt M.S., Callaway C.W., Carson A.P., Chamberlain A.M., Chang A.R., Cheng S., Delling F.N., et al. Heart Disease and Stroke Statistics-2020 Update: A Report From the American Heart Association. Circulation. 2020;141:e139–e596. doi: 10.1161/CIR.0000000000000757. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

