Naxitamab Combined with Granulocyte-Macrophage Colony-Stimulating Factor as Consolidation for High-Risk Neuroblastoma Patients in First Complete Remission under Compassionate Use-Updated Outcome Report
- PMID: 37174002
- PMCID: PMC10177429
- DOI: 10.3390/cancers15092535
Naxitamab Combined with Granulocyte-Macrophage Colony-Stimulating Factor as Consolidation for High-Risk Neuroblastoma Patients in First Complete Remission under Compassionate Use-Updated Outcome Report
Abstract
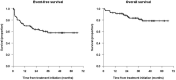
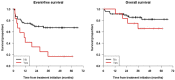
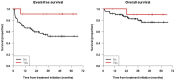
Naxitamab is an anti-GD2 antibody approved for the treatment of relapsed/refractory HR-NB. We report the survival, safety, and relapse pattern of a unique set of HR-NB patients consolidated with naxitamab after having achieved first CR. Eighty-two patients were treated with 5 cycles of GM-CSF for 5 days at 250 μg/m2/day (-4 to 0), followed by GM-CSF for 5 days at 500 μg/m2/day (1-5) and naxitamab at 3 mg/kg/day (1, 3, 5), on an outpatient basis. All patients but one were older than 18 months at diagnosis and had stage M; 21 (25.6%) pts had MYCN-amplified (A) NB; and 12 (14.6%) detectable MRD in the BM. Eleven (13.4%) pts had received high-dose chemotherapy and ASCT and 26 (31.7%) radiotherapy before immunotherapy. With a median follow-up of 37.4 months, 31 (37.8%) pts have relapsed. The pattern of relapse was predominantly (77.4%) an isolated organ. Five-year EFS and OS were 57.9% (71.4% for MYCN A) 95% CI = (47.2, 70.9%); and 78.6% (81% for MYCN A) 95% CI = (68.7%, 89.8%), respectively. EFS showed significant differences for patients having received ASCT (p = 0.037) and pre-immunotherapy MRD (p = 0.0011). Cox models showed only MRD as a predictor of EFS. In conclusion, consolidation with naxitamab resulted in reassuring survival rates for HR-NB patients after end-induction CR.
Keywords: GM-CSF; anti-GD2 immunotherapy; consolidation; high-risk; naxitamab; neuroblastoma.
Conflict of interest statement
J.M. declares consulting fees from Ymabs Therapeutics. The rest of the authors declare no conflict of interest.
Figures
Similar articles
-
Naxitamab combined with granulocyte-macrophage colony-stimulating factor as consolidation for high-risk neuroblastoma patients in complete remission.Pediatr Blood Cancer. 2021 Oct;68(10):e29121. doi: 10.1002/pbc.29121. Epub 2021 May 22. Pediatr Blood Cancer. 2021. PMID: 34022112
-
MYCN-amplified stage 2/3 neuroblastoma: excellent survival in the era of anti-GD2 immunotherapy.Oncotarget. 2017 Aug 24;8(56):95293-95302. doi: 10.18632/oncotarget.20513. eCollection 2017 Nov 10. Oncotarget. 2017. PMID: 29221128 Free PMC article.
-
The Role of Autologous Stem-Cell Transplantation in High-Risk Neuroblastoma Consolidated by anti-GD2 Immunotherapy. Results of Two Consecutive Studies.Front Pharmacol. 2020 Oct 30;11:575009. doi: 10.3389/fphar.2020.575009. eCollection 2020. Front Pharmacol. 2020. PMID: 33324208 Free PMC article.
-
How we approach the treatment of patients with high-risk neuroblastoma with naxitamab: experience from the Hospital Sant Joan de Déu in Barcelona, Spain.ESMO Open. 2022 Apr;7(2):100462. doi: 10.1016/j.esmoop.2022.100462. Epub 2022 Apr 6. ESMO Open. 2022. PMID: 35397431 Free PMC article. Review.
-
Naxitamab: a humanized anti-glycolipid disialoganglioside (anti-GD2) monoclonal antibody for treatment of neuroblastoma.Drugs Today (Barc). 2021 Nov;57(11):677-688. doi: 10.1358/dot.2021.57.11.3343691. Drugs Today (Barc). 2021. PMID: 34821881 Review.
Cited by
-
Neuroblastoma-A Review of Combination Immunotherapy.Int J Mol Sci. 2024 Jul 15;25(14):7730. doi: 10.3390/ijms25147730. Int J Mol Sci. 2024. PMID: 39062971 Free PMC article. Review.
-
GD2 in Breast Cancer: A Potential Biomarker and Therapeutic Target.Cancer Genomics Proteomics. 2024 Nov-Dec;21(6):549-556. doi: 10.21873/cgp.20471. Cancer Genomics Proteomics. 2024. PMID: 39467630 Free PMC article. Review.
-
Survival Benefit of Myeloablative Therapy with Autologous Stem Cell Transplantation in High-Risk Neuroblastoma: A Systematic Literature Review.Target Oncol. 2024 Mar;19(2):143-159. doi: 10.1007/s11523-024-01033-4. Epub 2024 Feb 24. Target Oncol. 2024. PMID: 38401028 Free PMC article.
-
Management of High-Risk Neuroblastoma with Soft-Tissue-Only Disease in the Era of Anti-GD2 Immunotherapy.Cancers (Basel). 2024 Apr 29;16(9):1735. doi: 10.3390/cancers16091735. Cancers (Basel). 2024. PMID: 38730688 Free PMC article.
-
GD2-targeting therapy: a comparative analysis of approaches and promising directions.Front Immunol. 2024 Mar 15;15:1371345. doi: 10.3389/fimmu.2024.1371345. eCollection 2024. Front Immunol. 2024. PMID: 38558810 Free PMC article. Review.
References
-
- Dini G., Philip T., Hartmann O., Pinkerton R., Chauvin F., Garaventa A., Lanino E., Dallorso S. Bone marrow transplantation for neuroblastoma: A review of 509 cases. Bone Marrow Transplant. 1989;4:42–46. - PubMed
-
- Dini G., Lanino E., Garaventa A., Rogers D., Dallorso S., Viscoli C., Castagnola E., Manno G., Brisigotti M., Rosanda C. Myeloablative therapy and unpurged autologous bone marrow transplantation for poor-prognosis neuroblastoma: Report of 34 cases. J. Clin. Oncol. 1991;9:962–969. doi: 10.1200/JCO.1991.9.6.962. - DOI - PubMed
-
- Philip T., Zucker J.M., Bernard J.L., Lutz P., Bordigoni P., Plouvier E., Robert A., Roché H., Souillet G., Bouffet E. Improved survival at 2 and 5 years in the LMCE1 unselected group of 72 children with stage IV neuroblastoma older than 1 year of age at diagnosis: Is cure possible in a small subgroup? J. Clin. Oncol. 1991;9:1037–1044. doi: 10.1200/JCO.1991.9.6.1037. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

