Efficacy and Safety of Osilodrostat in Paraneoplastic Cushing Syndrome: A Real-World Multicenter Study in France
- PMID: 36470583
- PMCID: PMC10188310
- DOI: 10.1210/clinem/dgac691
Efficacy and Safety of Osilodrostat in Paraneoplastic Cushing Syndrome: A Real-World Multicenter Study in France
Abstract
Context: Prospective studies have demonstrated the efficacy of osilodrostat in Cushing disease. No study has evaluated osilodrostat in a series of patients with paraneoplastic Cushing syndrome/ectopic adrenocorticotropin syndrome (PNCS/EAS).
Objective: This work aimed to evaluate in France the real-world efficacy and safety of osilodrostat in patients with PNCS/EAS.
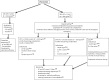
Methods: A total of 33 patients with PNCS/EAS with intense/severe hypercortisolism were involved in this retrospective, multicenter, real-world study. Patients received osilodrostat between May 2019 and March 2022 at a median initial dose (range) of 4 mg/day (1-60) and maximum dose, 20 mg/day (4-100), first under patient then cohort temporary authorizations and after marketing authorization. Regimens used titration (n = 6), block and replace (n = 16), or titration followed by block and replace (n = 11).
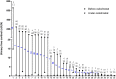
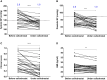
Results: In 11 patients receiving osilodrostat as first-line monotherapy, median 24-hour urinary free cortisol (24h-UFC) decreased dramatically (from 26 × upper limit of normal [ULN; 2.9-659] to 0.11 × ULN [0.08-14.9]; P < .001). In 9 of them, 24h-UFC normalization was achieved in 2 weeks (median). Thirteen additional patients were previously treated with classic steroidogenesis inhibitors but 10 of these 13 were not controlled. In these patients, osilodrostat monotherapy, used as second line, induced a significantly decreased of 24h-UFC (from 2.6 × ULN [1.1-144] to 0.22 × ULN [0.12-0.66]; P < .01). Nine additional patients received osilodrostat in combination with another anticortisolic drug, decreasing 24h-UFC from 11.8 × ULN (0.3-247) to 0.43 × ULN (0.33-2.4) (P < .01). In parallel, major clinical symptoms/comorbidities improved dramatically with improvement in blood pressure, hyperglycemia, and hypokalemia, allowing the discontinuation or dose reduction of patient treatments. Adrenal insufficiency (grade 3-4) was reported in 8 of 33 patients.
Conclusion: Osilodrostat is a rapidly efficient therapy for PNCS/EAS with severe/intense hypercortisolism. Osilodrostat was generally well tolerated; adrenal insufficiency was the main side effect.
Keywords: Cushing syndrome; adrenal insufficiency; corticotropin; ectopic adrenocorticotropic hormone syndrome; hypokalemia; neuroendocrine tumors; osilodrostat; paraneoplastic Cushing syndrome; steroidogenesis inhibitors.
© The Author(s) 2022. Published by Oxford University Press on behalf of the Endocrine Society.
Figures

Similar articles
-
Efficacy and safety of osilodrostat in patients with Cushing's disease (LINC 3): a multicentre phase III study with a double-blind, randomised withdrawal phase.Lancet Diabetes Endocrinol. 2020 Sep;8(9):748-761. doi: 10.1016/S2213-8587(20)30240-0. Epub 2020 Jul 27. Lancet Diabetes Endocrinol. 2020. PMID: 32730798 Clinical Trial.
-
Randomized Trial of Osilodrostat for the Treatment of Cushing Disease.J Clin Endocrinol Metab. 2022 Jun 16;107(7):e2882-e2895. doi: 10.1210/clinem/dgac178. J Clin Endocrinol Metab. 2022. PMID: 35325149 Free PMC article. Clinical Trial.
-
Metyrapone Versus Osilodrostat in the Short-Term Therapy of Endogenous Cushing's Syndrome: Results From a Single Center Cohort Study.Front Endocrinol (Lausanne). 2022 Jun 13;13:903545. doi: 10.3389/fendo.2022.903545. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 35769081 Free PMC article.
-
Clinical Utility of Osilodrostat in Cushing's Disease: Review of Currently Available Literature.Drug Des Devel Ther. 2023 Apr 27;17:1303-1312. doi: 10.2147/DDDT.S315359. eCollection 2023. Drug Des Devel Ther. 2023. PMID: 37143705 Free PMC article. Review.
-
Osilodrostat: A Review of Recent Clinical Studies and Practical Recommendations for its Use in the Treatment of Cushing Disease.Endocr Pract. 2021 Sep;27(9):956-965. doi: 10.1016/j.eprac.2021.06.012. Epub 2021 Aug 10. Endocr Pract. 2021. PMID: 34389514 Review.
Cited by
-
Current and Emerging Pharmacological Therapies for Cushing's Disease.Curr Pharm Des. 2024;30(10):757-777. doi: 10.2174/0113816128290025240216110928. Curr Pharm Des. 2024. PMID: 38424426 Review.
-
A Case of an Ectopic ACTH-Producing Tumor With Adrenal Shrinkage During Osilodrostat Administration.JCEM Case Rep. 2024 Jan 27;2(2):luae008. doi: 10.1210/jcemcr/luae008. eCollection 2024 Feb. JCEM Case Rep. 2024. PMID: 38283731 Free PMC article.
-
Severe Cushing's syndrome from an ectopic adrenocorticotropic hormone-secreting neuroendocrine tumour treated by osilodrostat.Endocrinol Diabetes Metab Case Rep. 2023 Oct 11;2023(4):23-0076. doi: 10.1530/EDM-23-0076. Print 2023 Oct 1. Endocrinol Diabetes Metab Case Rep. 2023. PMID: 37855644 Free PMC article.
-
Paraneoplastic neurological syndromes of small cell lung cancer.Postep Psychiatr Neurol. 2024 Jun;33(2):80-92. doi: 10.5114/ppn.2024.141157. Epub 2024 Jul 11. Postep Psychiatr Neurol. 2024. PMID: 39119541 Free PMC article. Review.
-
Successful Management of Cushing Syndrome From Ectopic ACTH Secretion in an Adolescent With Osilodrostat.JCEM Case Rep. 2023 Aug 17;1(4):luad101. doi: 10.1210/jcemcr/luad101. eCollection 2023 Jul. JCEM Case Rep. 2023. PMID: 37908982 Free PMC article.
References
-
- Lacroix A, Feelders RA, Stratakis CA, Nieman LK. Cushing's syndrome. Lancet. 2015;386(9996) 913‐927. - PubMed
-
- Young J, Haissaguerre M, Viera-Pinto O, Chabre O, Baudin E, Tabarin A. Management of endocrine disease; Cushing’s syndrome due to ectopic ACTH secretion: an expert operational opinion. Eur J Endocrinol. 2020;182(4):R29‐R58. - PubMed
-
- Hayes AR, Grossman AB. The ectopic adrenocorticotropic hormone syndrome: rarely easy, always challenging. Endocrinol Metab Clin North Am. 2018;47(2):409‐425. - PubMed
-
- Torpy DJ, Mullen N, Ilias I, Nieman LK. Association of hypertension and hypokalemia with Cushing's syndrome caused by ectopic ACTH secretion: a series of 58 cases. Ann N Y Acad Sci. 2002;970(1):134‐144. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

