Transcriptional factor OmpR positively regulates prodigiosin biosynthesis in Serratia marcescens FZSF02 by binding with the promoter of the prodigiosin cluster
- PMID: 36466667
- PMCID: PMC9712742
- DOI: 10.3389/fmicb.2022.1041146
Transcriptional factor OmpR positively regulates prodigiosin biosynthesis in Serratia marcescens FZSF02 by binding with the promoter of the prodigiosin cluster
Abstract
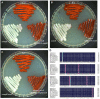
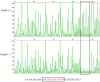
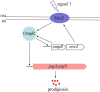
Prodigiosin is a promising secondary metabolite mainly produced by Serratia marcescens. The production of prodigiosin by S. marcescens is regulated by different kinds of regulatory systems, including the EnvZ/OmpR system. In this study, we demonstrated that the regulatory factor OmpR positively regulated prodigiosin production in S. marcescens FZSF02 by directly binding to the promoter region of the prodigiosin biosynthesis cluster with a lacZ reporter assay and electrophoretic mobility shift assay (EMSA). The binding sequence with the pig promoter was identified by a DNase I footprinting assay. We further demonstrate that OmpR regulates its own expression by directly binding to the promoter region of envZ/ompR. For the first time, the regulatory mechanism of prodigiosin production by the transcriptional factor OmpR was revealed.
Keywords: OmpR; Serratia marcescens; prodigiosin; regulatory mechanism; two-component system.
Copyright © 2022 Jia, Zhao, Liu, Lin, Lin and Chen.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Identification of Essential Genes Associated With Prodigiosin Production in Serratia marcescens FZSF02.Front Microbiol. 2021 Jul 22;12:705853. doi: 10.3389/fmicb.2021.705853. eCollection 2021. Front Microbiol. 2021. PMID: 34367107 Free PMC article.
-
LysR-Type Transcriptional Regulator MetR Controls Prodigiosin Production, Methionine Biosynthesis, Cell Motility, H2O2 Tolerance, Heat Tolerance, and Exopolysaccharide Synthesis in Serratia marcescens.Appl Environ Microbiol. 2020 Feb 3;86(4):e02241-19. doi: 10.1128/AEM.02241-19. Print 2020 Feb 3. Appl Environ Microbiol. 2020. PMID: 31791952 Free PMC article.
-
Improving prodigiosin production by transcription factor engineering and promoter engineering in Serratia marcescens.Front Microbiol. 2022 Aug 3;13:977337. doi: 10.3389/fmicb.2022.977337. eCollection 2022. Front Microbiol. 2022. PMID: 35992721 Free PMC article.
-
Recent advancements in high-level synthesis of the promising clinical drug, prodigiosin.Appl Microbiol Biotechnol. 2019 Feb;103(4):1667-1680. doi: 10.1007/s00253-018-09611-z. Epub 2019 Jan 12. Appl Microbiol Biotechnol. 2019. PMID: 30637495 Review.
-
Recent Advances in Prodigiosin as a Bioactive Compound in Nanocomposite Applications.Molecules. 2022 Aug 5;27(15):4982. doi: 10.3390/molecules27154982. Molecules. 2022. PMID: 35956931 Free PMC article. Review.
Cited by
-
Serratia marcescens ATCC 274 increases production of the red pigment prodigiosin in response to Chi phage infection.Sci Rep. 2024 Jul 31;14(1):17750. doi: 10.1038/s41598-024-68747-3. Sci Rep. 2024. PMID: 39085460 Free PMC article.
-
Prodigiosin: unveiling the crimson wonder - a comprehensive journey from diverse bioactivity to synthesis and yield enhancement.Front Microbiol. 2024 Jun 5;15:1412776. doi: 10.3389/fmicb.2024.1412776. eCollection 2024. Front Microbiol. 2024. PMID: 38903802 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources

