Antibody tests for identification of current and past infection with SARS-CoV-2
- PMID: 36394900
- PMCID: PMC9671206
- DOI: 10.1002/14651858.CD013652.pub2
Antibody tests for identification of current and past infection with SARS-CoV-2
Abstract
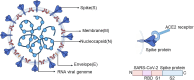
Background: The diagnostic challenges associated with the COVID-19 pandemic resulted in rapid development of diagnostic test methods for detecting SARS-CoV-2 infection. Serology tests to detect the presence of antibodies to SARS-CoV-2 enable detection of past infection and may detect cases of SARS-CoV-2 infection that were missed by earlier diagnostic tests. Understanding the diagnostic accuracy of serology tests for SARS-CoV-2 infection may enable development of effective diagnostic and management pathways, inform public health management decisions and understanding of SARS-CoV-2 epidemiology.
Objectives: To assess the accuracy of antibody tests, firstly, to determine if a person presenting in the community, or in primary or secondary care has current SARS-CoV-2 infection according to time after onset of infection and, secondly, to determine if a person has previously been infected with SARS-CoV-2. Sources of heterogeneity investigated included: timing of test, test method, SARS-CoV-2 antigen used, test brand, and reference standard for non-SARS-CoV-2 cases.
Search methods: The COVID-19 Open Access Project living evidence database from the University of Bern (which includes daily updates from PubMed and Embase and preprints from medRxiv and bioRxiv) was searched on 30 September 2020. We included additional publications from the Evidence for Policy and Practice Information and Co-ordinating Centre (EPPI-Centre) 'COVID-19: Living map of the evidence' and the Norwegian Institute of Public Health 'NIPH systematic and living map on COVID-19 evidence'. We did not apply language restrictions.
Selection criteria: We included test accuracy studies of any design that evaluated commercially produced serology tests, targeting IgG, IgM, IgA alone, or in combination. Studies must have provided data for sensitivity, that could be allocated to a predefined time period after onset of symptoms, or after a positive RT-PCR test. Small studies with fewer than 25 SARS-CoV-2 infection cases were excluded. We included any reference standard to define the presence or absence of SARS-CoV-2 (including reverse transcription polymerase chain reaction tests (RT-PCR), clinical diagnostic criteria, and pre-pandemic samples).
Data collection and analysis: We use standard screening procedures with three reviewers. Quality assessment (using the QUADAS-2 tool) and numeric study results were extracted independently by two people. Other study characteristics were extracted by one reviewer and checked by a second. We present sensitivity and specificity with 95% confidence intervals (CIs) for each test and, for meta-analysis, we fitted univariate random-effects logistic regression models for sensitivity by eligible time period and for specificity by reference standard group. Heterogeneity was investigated by including indicator variables in the random-effects logistic regression models. We tabulated results by test manufacturer and summarised results for tests that were evaluated in 200 or more samples and that met a modification of UK Medicines and Healthcare products Regulatory Agency (MHRA) target performance criteria.
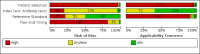
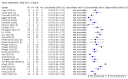
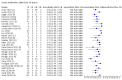
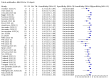
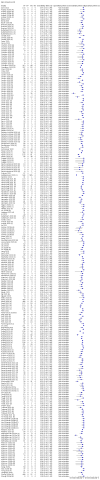
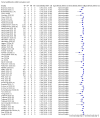
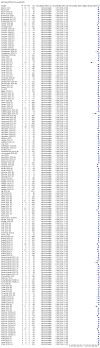
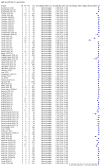
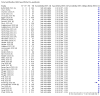
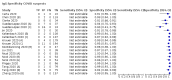
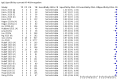
Main results: We included 178 separate studies (described in 177 study reports, with 45 as pre-prints) providing 527 test evaluations. The studies included 64,688 samples including 25,724 from people with confirmed SARS-CoV-2; most compared the accuracy of two or more assays (102/178, 57%). Participants with confirmed SARS-CoV-2 infection were most commonly hospital inpatients (78/178, 44%), and pre-pandemic samples were used by 45% (81/178) to estimate specificity. Over two-thirds of studies recruited participants based on known SARS-CoV-2 infection status (123/178, 69%). All studies were conducted prior to the introduction of SARS-CoV-2 vaccines and present data for naturally acquired antibody responses. Seventy-nine percent (141/178) of studies reported sensitivity by week after symptom onset and 66% (117/178) for convalescent phase infection. Studies evaluated enzyme-linked immunosorbent assays (ELISA) (165/527; 31%), chemiluminescent assays (CLIA) (167/527; 32%) or lateral flow assays (LFA) (188/527; 36%). Risk of bias was high because of participant selection (172, 97%); application and interpretation of the index test (35, 20%); weaknesses in the reference standard (38, 21%); and issues related to participant flow and timing (148, 82%). We judged that there were high concerns about the applicability of the evidence related to participants in 170 (96%) studies, and about the applicability of the reference standard in 162 (91%) studies. Average sensitivities for current SARS-CoV-2 infection increased by week after onset for all target antibodies. Average sensitivity for the combination of either IgG or IgM was 41.1% in week one (95% CI 38.1 to 44.2; 103 evaluations; 3881 samples, 1593 cases), 74.9% in week two (95% CI 72.4 to 77.3; 96 evaluations, 3948 samples, 2904 cases) and 88.0% by week three after onset of symptoms (95% CI 86.3 to 89.5; 103 evaluations, 2929 samples, 2571 cases). Average sensitivity during the convalescent phase of infection (up to a maximum of 100 days since onset of symptoms, where reported) was 89.8% for IgG (95% CI 88.5 to 90.9; 253 evaluations, 16,846 samples, 14,183 cases), 92.9% for IgG or IgM combined (95% CI 91.0 to 94.4; 108 evaluations, 3571 samples, 3206 cases) and 94.3% for total antibodies (95% CI 92.8 to 95.5; 58 evaluations, 7063 samples, 6652 cases). Average sensitivities for IgM alone followed a similar pattern but were of a lower test accuracy in every time slot. Average specificities were consistently high and precise, particularly for pre-pandemic samples which provide the least biased estimates of specificity (ranging from 98.6% for IgM to 99.8% for total antibodies). Subgroup analyses suggested small differences in sensitivity and specificity by test technology however heterogeneity in study results, timing of sample collection, and smaller sample numbers in some groups made comparisons difficult. For IgG, CLIAs were the most sensitive (convalescent-phase infection) and specific (pre-pandemic samples) compared to both ELISAs and LFAs (P < 0.001 for differences across test methods). The antigen(s) used (whether from the Spike-protein or nucleocapsid) appeared to have some effect on average sensitivity in the first weeks after onset but there was no clear evidence of an effect during convalescent-phase infection. Investigations of test performance by brand showed considerable variation in sensitivity between tests, and in results between studies evaluating the same test. For tests that were evaluated in 200 or more samples, the lower bound of the 95% CI for sensitivity was 90% or more for only a small number of tests (IgG, n = 5; IgG or IgM, n = 1; total antibodies, n = 4). More test brands met the MHRA minimum criteria for specificity of 98% or above (IgG, n = 16; IgG or IgM, n = 5; total antibodies, n = 7). Seven assays met the specified criteria for both sensitivity and specificity. In a low-prevalence (2%) setting, where antibody testing is used to diagnose COVID-19 in people with symptoms but who have had a negative PCR test, we would anticipate that 1 (1 to 2) case would be missed and 8 (5 to 15) would be falsely positive in 1000 people undergoing IgG or IgM testing in week three after onset of SARS-CoV-2 infection. In a seroprevalence survey, where prevalence of prior infection is 50%, we would anticipate that 51 (46 to 58) cases would be missed and 6 (5 to 7) would be falsely positive in 1000 people having IgG tests during the convalescent phase (21 to 100 days post-symptom onset or post-positive PCR) of SARS-CoV-2 infection.
Authors' conclusions: Some antibody tests could be a useful diagnostic tool for those in whom molecular- or antigen-based tests have failed to detect the SARS-CoV-2 virus, including in those with ongoing symptoms of acute infection (from week three onwards) or those presenting with post-acute sequelae of COVID-19. However, antibody tests have an increasing likelihood of detecting an immune response to infection as time since onset of infection progresses and have demonstrated adequate performance for detection of prior infection for sero-epidemiological purposes. The applicability of results for detection of vaccination-induced antibodies is uncertain.
Copyright © 2022 The Authors. Cochrane Database of Systematic Reviews published by John Wiley & Sons, Ltd. on behalf of The Cochrane Collaboration.
Conflict of interest statement
Tilly Fox: none known
Julia Geppert: none known
Jacqueline Dinnes: FIND (grant/contract); Cochrane DTA editor
Katie Scandrett: none known
Jacob Bigio: none known
Giorgia Sulis: none known
Dineshani Hettiarachchi: none known
Yasith Mathangasinghe: none known
Praveen Weeratunga: none known
Dakshitha Wickramasinghe: none known
Hanna Bergman: none known
Brian S Buckley: none known
Katrin Probyn: none known
Yanina Sguassero: Cochrane Response (employment); editor assistant of CDPLPG and Cochrane Clinical Answers.
Jane Cunningham: no relevant interests; affiliated to WHO, which produces guidance on use of SARS‐CoV‐2 rapid tests.
Sabine Dittrich: FIND (employment), the global alliance for diagnostic.
Devy Emperador: no relevant interests; employed by FIND with funding from FCDO and KFW. FIND is a global non‐for profit product development partnership and WHO Diagnostic Collaboration Centre. It is FIND’s role to accelerate access to high quality diagnostic tools for low‐resource settings and this is achieved by supporting both R&D and access activities for a wide range of diseases, including COVID‐19. FIND has several clinical research projects to evaluate multiple new diagnostic tests against published Target Product Profiles that have been defined through consensus processes. These studies are for diagnostic products developed by private sector companies who provide access to know how, equipment/reagents, and contribute through unrestricted donations as per FIND policy and external SAC review.
Lotty Hooft: no relevant interests; DTA Editorial Team; PMG implementation team.
Mariska MG Leeflang: no relevant interests; Diagnostic Test Accuracy Editorial Team member.
Matthew DF McInnes: no relevant interests; works as a health professional at the Ottawa Hospital.
René Spijker: none known
Thomas Struyf: none known
Ann Van den Bruel: none known
Jan Verbakel: none known
Clare Davenport: no relevant interests; Contact Editor for the Cochrane DTA Editorial Team; not involved in the editorial process for this review update because of this conflict of interest.
Yemisi Takwoingi: no relevant interests; Member, Cochrane Editorial Board; Editor, Cochrane Infectious Diseases Group; Statistical Editor, Cochrane BJMT Group; Cochrane DTA Editor.
Sian Taylor‐Phillips: finddx (grant/contract) ‐ funding to Warwick University from Birmingham University to fund a staff member time (approx 6 months part time) working on this review. No funding to individuals (funds originally came from FIND diagnostics, a charity); National Institute for Health Research (grant/contract) ‐ NIHR Career Development Fellowship NIHR‐CDF‐2016‐018 for methods of evaluating screening tests. Money to institution (University of Warwick); involved with the EDSAB‐HOME study, Public Health England, as co‐author but did not receive any funds for participation.
Jonathan J Deeks: no relevant interests; Eight podcasts, including Talk Evidence (BMJ), More‐or‐Less (Radio 4), Inside Science (Radio 4), The Newscast (Radio 4). Five opinion pieces in Guardian, unHerd and the BMJ. Numerous television, radio and mainstream media interviews giving substantial coverage of scientific issues related to test evaluation for COVID‐19. Presented evidence to the House of Lords Select Committee, and the All Parliamentary Party Investigation on COVID‐19. Two invited editorials on COVID‐19 for the BMJ; Cochrane DTA editor.
Figures
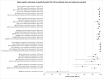



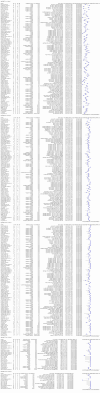



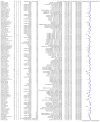







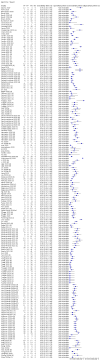





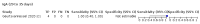
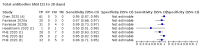
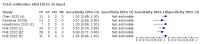
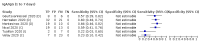
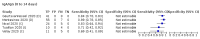
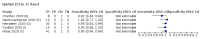
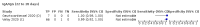
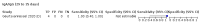
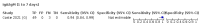
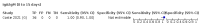








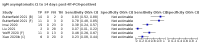
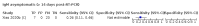
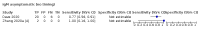
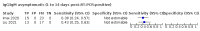
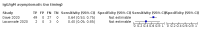
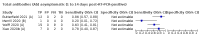
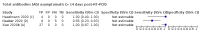
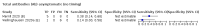
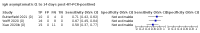
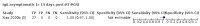


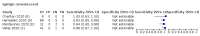

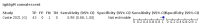


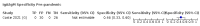
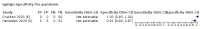

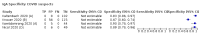

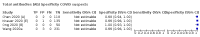





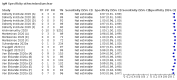
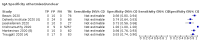
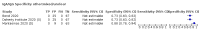
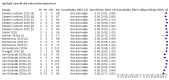
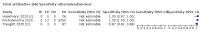
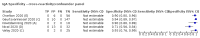

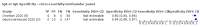


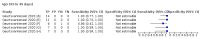
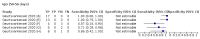
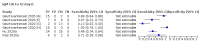
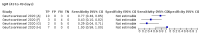
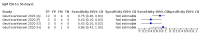
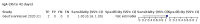
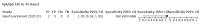
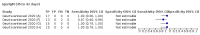
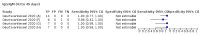
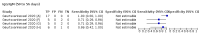
Update of
-
Antibody tests for identification of current and past infection with SARS-CoV-2.Cochrane Database Syst Rev. 2020 Jun 25;6(6):CD013652. doi: 10.1002/14651858.CD013652. Cochrane Database Syst Rev. 2020. Update in: Cochrane Database Syst Rev. 2022 Nov 17;11:CD013652. doi: 10.1002/14651858.CD013652.pub2. PMID: 32584464 Free PMC article. Updated.
Similar articles
-
The effect of sample site and collection procedure on identification of SARS-CoV-2 infection.Cochrane Database Syst Rev. 2024 Dec 16;12(12):CD014780. doi: 10.1002/14651858.CD014780. Cochrane Database Syst Rev. 2024. PMID: 39679851 Free PMC article. Review.
-
Defining the optimum strategy for identifying adults and children with coeliac disease: systematic review and economic modelling.Health Technol Assess. 2022 Oct;26(44):1-310. doi: 10.3310/ZUCE8371. Health Technol Assess. 2022. PMID: 36321689 Free PMC article.
-
RETRACTED: Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial.Int J Antimicrob Agents. 2020 Jul;56(1):105949. doi: 10.1016/j.ijantimicag.2020.105949. Epub 2020 Mar 20. Int J Antimicrob Agents. 2020. Retraction in: Int J Antimicrob Agents. 2025 Jan;65(1):107416. doi: 10.1016/j.ijantimicag.2024.107416. PMID: 32205204 Free PMC article. Retracted. Clinical Trial.
-
Accuracy of routine laboratory tests to predict mortality and deterioration to severe or critical COVID-19 in people with SARS-CoV-2.Cochrane Database Syst Rev. 2024 Aug 6;8(8):CD015050. doi: 10.1002/14651858.CD015050.pub2. Cochrane Database Syst Rev. 2024. PMID: 39105481 Free PMC article. Review.
-
Impact of residual disease as a prognostic factor for survival in women with advanced epithelial ovarian cancer after primary surgery.Cochrane Database Syst Rev. 2022 Sep 26;9(9):CD015048. doi: 10.1002/14651858.CD015048.pub2. Cochrane Database Syst Rev. 2022. PMID: 36161421 Free PMC article. Review.
Cited by
-
Comprehensive Review of COVID-19: Epidemiology, Pathogenesis, Advancement in Diagnostic and Detection Techniques, and Post-Pandemic Treatment Strategies.Int J Mol Sci. 2024 Jul 26;25(15):8155. doi: 10.3390/ijms25158155. Int J Mol Sci. 2024. PMID: 39125722 Free PMC article. Review.
-
Natural Antibodies Produced in Vaccinated Patients and COVID-19 Convalescents Recognize and Hydrolyze Oligopeptides Corresponding to the S-Protein of SARS-CoV-2.Vaccines (Basel). 2023 Sep 15;11(9):1494. doi: 10.3390/vaccines11091494. Vaccines (Basel). 2023. PMID: 37766170 Free PMC article.
-
BRET-based biosensors for SARS-CoV-2 oligonucleotide detection.Front Bioeng Biotechnol. 2024 Jun 3;12:1353479. doi: 10.3389/fbioe.2024.1353479. eCollection 2024. Front Bioeng Biotechnol. 2024. PMID: 38887615 Free PMC article.
-
A novel precision-serology assay for SARS-CoV-2 infection based on linear B-cell epitopes of Spike protein.Front Immunol. 2023 May 12;14:1166924. doi: 10.3389/fimmu.2023.1166924. eCollection 2023. Front Immunol. 2023. PMID: 37251407 Free PMC article.
-
The Use of Next-Generation Sequencing in Personalized Medicine.Methods Mol Biol. 2025;2866:287-315. doi: 10.1007/978-1-0716-4192-7_16. Methods Mol Biol. 2025. PMID: 39546209 Review.
References
References to studies included in this review
Adams 2020 {published data only}
-
- Adams ER, Augustin Y, Byrne RL, Clark DJ, Cocozza M, Cubas-Atienzar AI, et al. Rapid development of COVID-19 rapid diagnostics for low resource settings: accelerating delivery through transparency, responsiveness, and open collaboration. medRxiv [Preprint] 2020. [DOI: ]
Alvim 2020 {published data only}
-
- Alvim RGF, Lima TM, Rodrigues DAS, Marsili FF, Bozza VBT, Higa LM, et al. An affordable anti-SARS-CoV-2 spike ELISA test for early detection of IgG seroconversion suited for large-scale surveillance studies in low-income countries. medRxiv [Preprint] 2020. [DOI: ]
Andrey 2020a [A] {published data only}
Andrey 2020a [B] {published data only}
-
- See first entry for publication details for this study. Andrey 2020a [A].
Andrey 2020b [A] {published data only}
Andrey 2020b [B] {published data only}
-
- See first entry for publication details for this study. Andrey 2020b [A].
Andrey 2020b [C] {published data only}
-
- See first entry for publication details for this study. Andrey 2020b [A].
Bartolini 2020 [A] {published data only}
-
- Bartolini A, Scapaticci M, Bioli M, Lazzarotto T, Re MC, Mancini R. Immunochromatographic assays for COVID-19 epidemiological screening: our experience. medRxiv [Preprint] 2020. [DOI: ]
Bartolini 2020 [B] {published data only}
-
- See first entry for publication details for this study. Bartolini 2020 [A].
Beavis 2020 {published data only}
Bernasconi 2020 {published data only}
-
- Bernasconi L, Oberle M, Gisler V, Ottiger C, Fankhauser H, Schuetz P, et al. Diagnostic performance of a SARS-CoV-2 IgG/IgM lateral flow immunochromatography assay in symptomatic patients presenting to the emergency department. Clinical Chemistry and Laboratory Medicine 2020;58(9):e159-61. - PubMed
Bettencourt 2020 {published data only}
Bond 2020 {published data only}
Boukli 2020 [A] {published data only}
-
- Boukli N, Le Mene M, Schnuriger A, Cuervo NS, Laroche C, Morand-Joubert L, et al. High incidence of false-positive results in patients with acute infections other than COVID-19 by the Liaison SARS-CoV-2 commercial chemiluminescent microparticle immunoassay for detection of IgG Anti-SARS-CoV-2 antibodies. Journal of Clinical Microbiology 2020;58(11):e01352-20. - PMC - PubMed
Boulki 2020 [B] {published data only}
-
- See first entry for publication details for this study. Boukli 2020 [A].
Brochot 2020 [A] {published data only}
Brochot 2020 [B] {published data only}
-
- See first entry for publication details for this study. Brochot 2020 [A].
Brochot 2020 [C] {published data only}
-
- See first entry for publication details for this study. Brochot 2020 [A].
Brochot 2020 [D] {published data only}
-
- See first entry for publication details for this study. Brochot 2020 [A].
Brochot 2020 [E] {published data only}
-
- See first entry for publication details for this study. Brochot 2020 [A].
Bryan 2020a {published data only}
-
- Bryan A, Fink SL, Gattuso MA, Pepper G, Chaudhary A, Wener MH, et al. Anti-SARS-CoV-2 IgG antibodies are associated with reduced viral load. medRxiv [Preprint] 2020. [DOI: ]
Bundschuh 2020 {published data only}
Butterfield 2021 [A] {published data only}
Butterfield 2021 [B] {published data only}
-
- See first entry for publication details for this study. Butterfield 2021 [A].
Butterfield 2021 [C] {published data only}
-
- See first entry for publication details for this study. Butterfield 2021 [A].
Butterfield 2021 [D] {published data only}
-
- See first entry for publication details for this study. Butterfield 2021 [A].
Butterfield 2021 [E] {published data only}
-
- See first entry for publication details for this study. Butterfield 2021 [A].
Butterfield 2021 [F] {published data only}
-
- See first entry for publication details for this study. Butterfield 2021 [A].
Candel 2020 {published data only}
Carozzi 2020 [A] {published data only}
-
- Carozzi FM, Cusi MG, Pistello M, Galli L, Bartoloni A, Anichini G, et al. Detection of asymptomatic SARS-CoV-2 infections among healthcare workers: results from a large-scale screening program based on rapid serological testing. medRxiv [Preprint] 2020. [DOI: ]
Carozzi 2020 [B] {published data only}
-
- See first entry for publication details for this study. Carozzi 2020 [A].
Carta 2020 {published data only}
-
- Carta M, Bragagnolo L, Tramarin A, Barzon E, Cappelletti A, Pascarella M, et al. Anti SARS-CoV-2 antibodies monitoring in a group of residents in a long term care facility during COVID-19 pandemic peak. Diagnosis 2020;7(4):395-400. - PubMed
Case 2020 [A] {published data only}
Case 2020 [B] {published data only}
-
- See first entry for publication details for this study. Case 2020 [A].
Caturegli 2020 {published data only}
Cervia 2020 {published data only}
Chan 2020a {published data only}
Charlton 2020 [A] {published data only}
Charlton 2020 [B] {published data only}
-
- See first entry for publication details for this study. Charlton 2020 [A].
Charlton 2020 [C] {published data only}
-
- See first entry for publication details for this study. Charlton 2020 [A].
Charlton 2020 [D] {published data only}
-
- See first entry for publication details for this study. Charlton 2020 [A].
Charlton 2020 [E] {published data only}
-
- See first entry for publication details for this study. Charlton 2020 [A].
Charlton 2020 [F] {published data only}
-
- See first entry for publication details for this study. Charlton 2020 [A].
Charlton 2020 [G] {published data only}
-
- See first entry for publication details for this study. Charlton 2020 [A].
Charlton 2020 [H] {published data only}
-
- See first entry for publication details for this study. Charlton 2020 [A].
Charlton 2020 [I] {published data only}
-
- See first entry for publication details for this study. Charlton 2020 [A].
Charlton 2020 [J] {published data only}
-
- See first entry for publication details for this study. Charlton 2020 [A].
Charlton 2020 [K] {published data only}
-
- See first entry for publication details for this study. Charlton 2020 [A].
Charlton 2020 [L] {published data only}
-
- See first entry for publication details for this study. Charlton 2020 [A].
Charpentier 2020 [A] {published data only}
Charpentier 2020 [B] {published data only}
-
- See first entry for publication details for this study. Charpentier 2020 [A].
Charpentier 2020 [C] {published data only}
-
- See first entry for publication details for this study. Charpentier 2020 [A].
Chaudhuri 2020 [A] {published data only}
Chaudhuri 2020 [B] {published data only}
-
- See first entry for publication details for this study. Chaudhuri 2020 [A].
Chen 2020 [A] {published data only}
-
- Chen SY, Lee YL, Lin YC, Lee NY, Liao CH, Hung YP, et al. Multicenter evaluation of two chemiluminescence and three lateral flow immunoassays for the diagnosis of COVID-19 and assessment of antibody dynamic responses to SARS-CoV-2 in Taiwan. Emerging Microbes & Infections 2020;9(1):2157-68. - PMC - PubMed
Chen 2020 [B] {published data only}
-
- See first entry for publication details for this study. Chen 2020 [A].
Chen 2020 [C] {published data only}
-
- See first entry for publication details for this study. Chen 2020 [A].
Chen 2020 [D] {published data only}
-
- See first entry for publication details for this study. Chen 2020 [A].
Chen 2020 [E] {published data only}
-
- See first entry for publication details for this study. Chen 2020 [A].
Chew 2020 {published data only}
Chong 2021 {published data only}
Clarke 2020 {published data only}
Conklin 2020 [A] {published data only}
Conklin 2020 [B] {published data only}
-
- See first entry for publication details for this study. Conklin 2020 [A].
Conklin 2020 [C] {published data only}
-
- See first entry for publication details for this study. Conklin 2020 [A].
Conklin 2020 [D] {published data only}
-
- See first entry for publication details for this study. Conklin 2020 [A].
Conklin 2020 [E] {published data only}
-
- See first entry for publication details for this study. Conklin 2020 [A].
Conklin 2020 [F] {published data only}
-
- See first entry for publication details for this study. Conklin 2020 [A].
Conklin 2020 [G] {published data only}
-
- See first entry for publication details for this study. Conklin 2020 [A].
Conklin 2020 [H] {published data only}
-
- See first entry for publication details for this study. Conklin 2020 [A].
Conklin 2020 [I] {published data only}
-
- See first entry for publication details for this study. Conklin 2020 [A].
Conklin 2020 [J] {published data only}
-
- See first entry for publication details for this study. Conklin 2020 [A].
Conklin 2020 [K] {published data only}
-
- See first entry for publication details for this study. Conklin 2020 [A].
Conklin 2020 [L] {published data only}
-
- See first entry for publication details for this study. Conklin 2020 [A].
Conklin 2020 [M] {published data only}
-
- See first entry for publication details for this study. Conklin 2020 [A].
Conklin 2020 [N] {published data only}
-
- See first entry for publication details for this study. Conklin 2020 [A].
Conklin 2020 [O] {published data only}
-
- See first entry for publication details for this study. Conklin 2020 [A].
Conklin 2020 [P] {published data only}
-
- See first entry for publication details for this study. Conklin 2020 [A].
Costa 2020 {published data only}
Coste 2021 [A] {published data only}
Coste 2021 [B] {published data only}
-
- See first entry for publication details for this study. Coste 2021 [A].
Coste 2021 [C] {published data only}
-
- See first entry for publication details for this study. Coste 2021 [A].
Coste 2021 [D] {published data only}
-
- See first entry for publication details for this study. Coste 2021 [A].
Coste 2021 [E] {published data only}
-
- See first entry for publication details for this study. Coste 2021 [A].
Coste 2021 [F] {published data only}
-
- See first entry for publication details for this study. Coste 2021 [A].
Coste 2021 [G] {published data only}
-
- See first entry for publication details for this study. Coste 2021 [A].
Coste 2021 [H] {published data only}
-
- See first entry for publication details for this study. Coste 2021 [A].
Coste 2021 [I] {published data only}
-
- See first entry for publication details for this study. Coste 2021 [A].
Coste 2021 [J] {published data only}
-
- See first entry for publication details for this study. Coste 2021 [A].
Coste 2021 [K] {published data only}
-
- See first entry for publication details for this study. Coste 2021 [A].
Coste 2021 [L] {published data only}
-
- See first entry for publication details for this study. Coste 2021 [A].
Coste 2021 [M] {published data only}
-
- See first entry for publication details for this study. Coste 2021 [A].
Coste 2021 [N] {published data only}
-
- See first entry for publication details for this study. Coste 2021 [A].
Coste 2021 [O] {published data only}
-
- See first entry for publication details for this study. Coste 2021 [A].
Criscuolo 2020 [A] {published data only}
-
- Criscuolo E, Diotti RA, Strollo M, Rolla S, Ambrosi A, Locatelli M, et al. Poor correlation between antibody titers and neutralizing activity in sera from SARS-CoV-2 infected subjects. medRxiv [Preprint] 2020. [https://doi.org/10.1101/2020.07.10.20150375] - PMC - PubMed
Criscuolo 2020 [B] {published data only}
-
- See first entry for publication details for this study. Criscuolo 2020 [A].
Dave 2020 {published data only}
-
- Dave M, Poswal L, Bedi V, Regar L, Vijayvargiya R, Sharma M, et al. Study of antibody-based rapid card test in COVID-19 patients admitted in a tertiary care COVID hospital in Southern Rajasthan. Journal, Indian Academy of Clinical Medicine 2020;21(1-2):7-11.
Decru 2020 [A] {published data only}
-
- Decru B, Van Elslande J, Weemaes M, Houben E, Empsen I, Andre E, et al. Comparison of the diagnostic performance with whole blood and plasma of four rapid antibody tests for SARS-CoV-2. Clinical Chemistry and Laboratory Medicine 2020;58(10):e197-9. - PubMed
Decru 2020 [B] {published data only}
-
- See first entry for publication details for this study. Decru 2020 [A].
Decru 2020 [C] {published data only}
-
- See first entry for publication details for this study. Decru 2020 [A].
Decru 2020 [D] {published data only}
-
- See first entry for publication details for this study. Decru 2020 [A].
Delliere 2020 [A] {published data only}
Delliere 2020 [B] {published data only}
-
- See first entry for publication details for this study. Delliere 2020 [A].
Doherty Institute 2020 [A] {published data only}
-
- Doherty Institute. Post-market validation of a further three serological assays for COVID-19 (10th Aug 2020). Report prepared for Office of Health Protection, Commonwealth Government of Australia; The Therapeutics Goods Administration (TGA) of Australia. https://www.health.gov.au/resources/publications/post-market-validation-....
-
- Doherty Institute. Post-market validation of serological assays for COVID-19 - Updated Report (2 June 2020). Report prepared for: Office of Health Protection, Commonwealth Government of Australia; The Therapeutics Goods Administration (TGA) of Australia. https://www.health.gov.au/resources/publications/post-market-validation-....
-
- Doherty Institute. Post-market validation of three serological assays for COVID-19 (29th April 2020). Report prepared for: Office of Health Protection, Commonwealth Government of Australia; The Therapeutics Goods Administration (TGA) of Australia. https://www.health.gov.au/resources/publications/post-market-validation-....
Doherty Institute 2020 [B] {published data only}
-
- See first entry for publication details for this study. Doherty Institute 2020 [A].
Doherty Institute 2020 [C] {published data only}
-
- See first entry for publication details for this study. Doherty Institute 2020 [A].
Doherty Institute 2020 [D] {published data only}
-
- See first entry for publication details for this study. Doherty Institute 2020 [A].
Doherty Institute 2020 [E] {published data only}
-
- See first entry for publication details for this study. Doherty Institute 2020 [A].
Doherty Institute 2020 [F] {published data only}
-
- See first entry for publication details for this study. Doherty Institute 2020 [A].
Doherty Institute 2020 [G] {published data only}
-
- See first entry for publication details for this study. Doherty Institute 2020 [A].
DomBourian 2020 [A] {published data only}
DomBourian 2020 [B] {published data only}
-
- See first entry for publication details for this study. DomBourian 2020 [A].
Dora 2020 {published data only}
-
- Dora AV, Winnett A, Fulcher JA, Sohn L, Calub F, Lee-Chang I, et al. Using serologic testing to assess the effectiveness of outbreak control efforts, serial PCR testing, and cohorting of positive SARS-CoV-2 patients in a skilled nursing facility. Clinical Infectious Diseases 2021;73:545-48. [DOI: 10.1093/cid/ciaa1286] - DOI - PMC - PubMed
Dortet 2020 {published data only}
Dortet 2021 [A] {published data only}
-
- Dortet L, Ronat JB, Vauloup-Fellous C, Langendorf C, Mendels DA, Emeraud C, et al. Evaluating 10 commercially available sars-cov-2 rapid serological tests by use of the STARD (Standards for reporting of diagnostic accuracy studies) method. Journal of Clinical Microbiology 2021;59(2):e02342-20. - PMC - PubMed
Dortet 2021 [B] {published data only}
-
- See first entry for publication details for this study. Dortet 2021 [A].
Du 2021 {published data only}
Egger 2020 [A] {published data only}
Egger 2020 [B] {published data only}
-
- See first entry for publication details for this study. Egger 2020 [A].
Fafi‐Kremer 2020 {published data only}
Favresse 2020a {published data only}
Favresse 2020b {published data only}
-
- Favresse J, Eucher C, Elsen M, Laffineur K, Dogne JM, Douxfils J. Response of anti-SARS-CoV-2 total antibodies to nucleocapsid antigen in COVID-19 patients: a longitudinal study. Clinical Chemistry and Laboratory Medicine 2020;58(10):e193-6. - PubMed
Fenwick 2021 [A] {published data only}
Fenwick 2021 [B] {published data only}
-
- See first entry for publication details for this study. Fenwick 2021 [A].
Fenwick 2021 [C] {published data only}
-
- See first entry for publication details for this study. Fenwick 2021 [A].
Fenwick 2021 [D] {published data only}
-
- See first entry for publication details for this study. Fenwick 2021 [A].
Fenwick 2021 [E] {published data only}
-
- See first entry for publication details for this study. Fenwick 2021 [A].
Flinck 2021 [A] {published data only}
Flinck 2021 [B] {published data only}
-
- See first entry for publication details for this study. Flinck 2021 [A].
Flower 2020 [A] {published data only}
Flower 2020 [B] {published data only}
Flower 2020 [C] {published data only}
Flower 2020 [D] {published data only}
Flower 2020 [E] {published data only}
Fragkou 2020 {published data only}
Fujigaki 2020 [A] {published data only}
Fujigaki 2020 [B] {published data only}
-
- See first entry for publication details for this study. Fujigaki 2020 [A].
Fujigaki 2020 [C] {published data only}
-
- See first entry for publication details for this study. Fujigaki 2020 [A].
Gao 2020a {published data only}
-
- Gao Y, Yuan Y, Li TT, Wang WX, Li YX, Li A, et al. Evaluation of the auxiliary diagnosis value of antibodies assays for the detection of novel coronavirus (SARS-Cov-2). medRxiv [Preprint] 2020. [DOI: ]
Gao 2020b [A] {published data only}
Gao 2020b [B] {published data only}
-
- See first entry for publication details for this study. Gao 2020b [A].
Gao 2020b [C] {published data only}
-
- See first entry for publication details for this study. Gao 2020b [A].
Garnett 2020 {published data only}
GeurtsvanKessel 2020 [A] {published data only}
GeurtsvanKessel 2020 [B] {published data only}
-
- See first entry for publication details for this study. GeurtsvanKessel 2020 [A].
GeurtsvanKessel 2020 [C] {published data only}
-
- See first entry for publication details for this study. GeurtsvanKessel 2020 [A].
GeurtsvanKessel 2020 [D] {published data only}
-
- See first entry for publication details for this study. GeurtsvanKessel 2020 [A].
GeurtsvanKessel 2020 [E] {published data only}
-
- See first entry for publication details for this study. GeurtsvanKessel 2020 [A].
GeurtsvanKessel 2020 [F] {published data only}
-
- See first entry for publication details for this study. GeurtsvanKessel 2020 [A].
GeurtsvanKessel 2020 [G] {published data only}
-
- See first entry for publication details for this study. GeurtsvanKessel 2020 [A].
GeurtsvanKessel 2020 [H] {published data only}
-
- See first entry for publication details for this study. GeurtsvanKessel 2020 [A].
Graham 2021 {published data only}
Gudbjartsson 2020 [A] {published data only}
Gudbjartsson 2020 [B] {published data only}
-
- See first entry for publication details for this study. Gudbjartsson 2020 [A].
Gudbjartsson 2020 [C] {published data only}
-
- See first entry for publication details for this study. Gudbjartsson 2020 [A].
Guedez‐Lopez 2020 [A] {published data only}
-
- Guedez-Lopez GV, Alguacil-Guillen M, Gonzalez-Donapetry P, Bloise I, Tornero-Marin C, Gonzalez-Garcia J, et al. Evaluation of three immunochromatographic tests for rapid detection of antibodies against SARS-CoV-2. European Journal of Clinical Microbiology and Infectious Diseases 2020;39(12):2289-97. - PMC - PubMed
Guedez‐Lopez 2020 [B] {published data only}
-
- See first entry for publication details for this study. Guedez-Lopez 2020 [A].
Guedez‐Lopez 2020 [C] {published data only}
-
- See first entry for publication details for this study. Guedez-Lopez 2020 [A].
Haljasmagi 2020 {published data only}
Hamilton 2020 {published data only}
Harritshoej 2021 [A] {published data only}
-
- Harritshøj LH, Gybel-Brask M, Afzal S, Kamstrup PR, Jørgensen CS, Thomsen MK, et al. Comparison of sixteen serological SARS-CoV-2 immunoassays in sixteen clinical laboratories. medRxiv [Preprint] 2020. [DOI: ]
Harritshoej 2021 [B] {published data only}
-
- See first entry for publication details for this study. Harritshoej 2021 [A].
Harritshoej 2021 [C] {published data only}
-
- See first entry for publication details for this study. Harritshoej 2021 [A].
Harritshoej 2021 [D] {published data only}
-
- See first entry for publication details for this study. Harritshoej 2021 [A].
Harritshoej 2021 [E] {published data only}
-
- See first entry for publication details for this study. Harritshoej 2021 [A].
Harritshoej 2021 [F] {published data only}
-
- See first entry for publication details for this study. Harritshoej 2021 [A].
Harritshoej 2021 [G] {published data only}
-
- See first entry for publication details for this study. Harritshoej 2021 [A].
Harritshoej 2021 [H] {published data only}
-
- See first entry for publication details for this study. Harritshoej 2021 [A].
Harritshoej 2021 [I] {published data only}
-
- See first entry for publication details for this study. Harritshoej 2021 [A].
Harritshoej 2021 [J] {published data only}
-
- See first entry for publication details for this study. Harritshoej 2021 [A].
Harritshoej 2021 [K] {published data only}
-
- See first entry for publication details for this study. Harritshoej 2021 [A].
Harritshoej 2021 [L] {published data only}
-
- See first entry for publication details for this study. Harritshoej 2021 [A].
Harritshoej 2021 [M] {published data only}
-
- See first entry for publication details for this study. Harritshoej 2021 [A].
Haselmann 2020 [A] {published data only}
Haselmann 2020 [B] {published data only}
-
- See first entry for publication details for this study. Haselmann 2020 [A].
Haselmann 2020 [C] {published data only}
-
- See first entry for publication details for this study. Haselmann 2020 [A].
Herroelen 2020 [A] {published data only}
Herroelen 2020 [B] {published data only}
-
- See first entry for publication details for this study. Herroelen 2020 [A].
Herroelen 2020 [C] {published data only}
-
- See first entry for publication details for this study. Herroelen 2020 [A].
Herroelen 2020 [D] {published data only}
-
- See first entry for publication details for this study. Herroelen 2020 [A].
Herroelen 2020 [E] {published data only}
-
- See first entry for publication details for this study. Herroelen 2020 [A].
Herroelen 2020 [F] {published data only}
-
- See first entry for publication details for this study. Herroelen 2020 [A].
Herroelen 2020 [G] {published data only}
-
- See first entry for publication details for this study. Herroelen 2020 [A].
Hoffman 2020 {published data only}
Hogan 2020a [A] {published data only}
-
- Hogan KO, Klippel D, Plapp FV, Liesman RM. Comparative Evaluation of Three Serologic Assays for the Identification of SARS-CoV-2 Antibodies. medRxiv [Preprint] 2020. [DOI: ]
Hogan 2020a [B] {published data only}
-
- See first entry for publication details for this study. Hogan 2020a [A].
Hogan 2020a [C] {published data only}
-
- See first entry for publication details for this study. Hogan 2020a [A].
Horber 2020 [A] {published data only}
-
- Horber S, Soldo J, Relker L, Jurgens S, Guther J, Peter S, et al. Evaluation of three fully-automated SARS-CoV-2 antibody assays. Clinical Chemistry and Laboratory Medicine 2020;58(12):2113-20. - PubMed
Horber 2020 [B] {published data only}
-
- See first entry for publication details for this study. Horber 2020 [A].
Horber 2020 [C] {published data only}
-
- See first entry for publication details for this study. Horber 2020 [A].
Hu 2020a {published data only}
-
- Hu Q, Cui X, Liu X, Peng B, Jiang J, Wang X, et al. The production of antibodies for SARS-CoV-2 and its clinical implication. medRxiv [Preprint] 2020. [DOI: ]
Hu 2020b [A] {published data only}
Hu 2020b [B] {published data only}
-
- See first entry for publication details for this study. Hu 2020b [A].
Hubbard 2021 [A] {published data only}
Hubbard 2021 [B] {published data only}
-
- See first entry for publication details for this study. Hubbard 2021 [A].
Imai 2020 {published data only}
Jaaskelainen 2020 {published data only}
Jin 2020 {published data only}
Jung 2020a {published data only}
Kaltenbach 2020 [A] {published data only}
-
- Kaltenbach H-M, Rudolf F, Linnik J, Deichmann J, Ruf T, Altamura R, et al. Initial characterisation of ELISA assays and the immune response of the clinically correlated SARS-CoV-2 biobank SERO-BL-COVID-19 collected during the pandemic onset in Switzerland. medRxiv [Preprint] 2020. [https://doi.org/10.1101/2020.07.05.20145888]
Kaltenbach 2020 [B] {published data only}
-
- See first entry for publication details for this study. Kaltenbach 2020 [A].
Kaltenbach 2020 [C] {published data only}
-
- See first entry for publication details for this study. Kaltenbach 2020 [A].
Kaneko 2021 {published data only}
-
- Kaneko S, Nukui Y, Arashiro T, Aiso Y, Sugii M, Hadano Y, et al. Clinical validation of an immunochromatographic SARS-Cov-2 IgM/IgG antibody assay with Japanese cohort. Journal of Medical Virology 2021;93(1):569-72. - PubMed
Knauer 2020 [A] {published data only}
Knauer 2020 [B] {published data only}
-
- See first entry for publication details for this study. Knauer 2020 [A].
Knauer 2020 [C] {published data only}
-
- See first entry for publication details for this study. Knauer 2020 [A].
Knauer 2020 [D] {published data only}
-
- See first entry for publication details for this study. Knauer 2020 [A].
Knauer 2020 [E] {published data only}
-
- See first entry for publication details for this study. Knauer 2020 [A].
Ko 2021 {published data only}
Kohmer 2020a [A] {published data only}
Kohmer 2020a [B] {published data only}
-
- See first entry for publication details for this study. Kohmer 2020a [A].
Kohmer 2020a [C] {published data only}
-
- See first entry for publication details for this study. Kohmer 2020a [A].
Kohmer 2020b [A] {published data only}
Kohmer 2020b [B] {published data only}
-
- See first entry for publication details for this study. Kohmer 2020b [A].
Kohmer 2020b [C] {published data only}
-
- See first entry for publication details for this study. Kohmer 2020b [A].
Kohmer 2020b [D] {published data only}
-
- See first entry for publication details for this study. Kohmer 2020b [A].
Kohmer 2020b [E] {published data only}
-
- See first entry for publication details for this study. Kohmer 2020b [A].
Kohmer 2020b [F] {published data only}
-
- See first entry for publication details for this study. Kohmer 2020b [A].
Korte 2021 [A] {published data only}
Korte 2021 [B] {published data only}
-
- See first entry for publication details for this study. Korte 2021 [A].
Korte 2021 [C] {published data only}
-
- See first entry for publication details for this study. Korte 2021 [A].
Kowitdamrong 2020 [A] {published data only}
-
- Kowitdamrong E, Puthanakit T, Jantarabenjakul W, Prompetchara E, Suchartlikitwong P, Putcharoen O, et al. Antibody responses to SARS-CoV-2 in coronavirus diseases 2019 patients with different severity. medRxiv [Preprint] 2020. [https://doi.org/10.1101/2020.09.06.20189480] - PMC - PubMed
Kowitdamrong 2020 [B] {published data only}
-
- See first entry for publication details for this study. Kowitdamrong 2020 [A].
Krishnamurthy 2020 {published data only}
Lassauniere 2020 [A] {published data only}
-
- Lassauniere R, Frische A, Harboe ZB, Nielsen AC, Fomsgaard A, Krogfelt KA, et al. Evaluation of nine commercial SARS-CoV-2 immunoassays. medRxiv [Preprint] 2020. [DOI: ]
Lassauniere 2020 [B] {published data only}
-
- See first entry for publication details for this study. Lassauniere 2020 [A].
Lassauniere 2020 [C] {published data only}
-
- See first entry for publication details for this study. Lassauniere 2020 [C].
Lassauniere 2020 [D] {published data only}
-
- See first entry for publication details for this study. Lassauniere 2020 [A].
Lassauniere 2020 [E] {published data only}
-
- See first entry for publication details for this study. Lassauniere 2020 [A].
Lassauniere 2020 [F] {published data only}
-
- See first entry for publication details for this study. Lassauniere 2020 [A].
Lassauniere 2020 [G] {published data only}
-
- See first entry for publication details for this study. Lassauniere 2020 [A].
Lau 2020a {published data only}
-
- Lau CS, Hoo SP, Yew SF, Ong SK, Lum LT, Heng PY, et al. Evaluation of the Roche Elecsys anti-SARS-CoV-2 assay. medRxiv [Preprint] 2020. [https://doi.org/10.1101/2020.06.28.20142232]
Lau 2020b {published data only}
-
- Lau CS, Hoo SP, Liang YL, Aw TC. Evaluation of the Abbott SARS-CoV-2 Ig-G assay. medRxiv [Preprint] 2020. [DOI: ]
Lau 2020c {published data only}
Lau 2020d {published data only}
Li 2020 [A] {published data only}
-
- Li B, Feng F, Yang G, Liu A, Yang N, Jiang Q, et al. Immunoglobulin G/M and cytokines detections in continuous sera from patients with novel coronaviruses (2019-nCoV) infection. SSRN [Preprint] 2020. [DOI: ]
Li 2020 [B] {published data only}
-
- See first entry for publication details for this study. Li 2020 [A].
Lippi 2020 [A] {published data only}
-
- Lippi G, Salvagno GL, Pegoraro M, Militello V, Caloi C, Peretti A, et al. Assessment of immune response to SARS-CoV-2 with fully automated MAGLUMI 2019-nCoV IgG and IgM chemiluminescence immunoassays. Clinical Chemistry and Laboratory Medicine 2020;58(7):1156-9. [DOI: 10.1515/cclm-2020-0473] - DOI - PubMed
Lippi 2020 [B] {published data only}
-
- See first entry for publication details for this study. Lippi 2020 [A].
Liu 2020a {published data only}
Liu 2020b [A] {published data only}
Liu 2020b [B] {published data only}
-
- See first entry for publication details for this study. Liu 2020b [A].
Liu 2020c {published data only}
Liu 2021 {published data only}
Loconsole 2020 {published data only}
-
- Loconsole D, Centrone F, Morcavallo C, Campanella S, Sallustio A, Quarto M, et al. The light and shadow of rapid serological tests for SARS-CoV-2 infection: results from a study in a large emergency department. International Journal of Environmental Research and Public Health 2020;17(18):6493. - PMC - PubMed
Long 2020 {published data only}
-
- Long Q, Deng H, Chen J, Hu J, Liu B, Liao P, et al. Antibody responses to SARS-CoV-2 in COVID-19 patients: the perspective application of serological tests in clinical practice. medRxiv [Preprint] 2020. [DOI: ]
-
- Long QX, Liu BZ, Deng HJ, Wu GC, Deng K, Chen YK, et al. Antibody responses to SARS-CoV-2 in patients with COVID-19. Nature Medicine 2020;26:845-8. - PubMed
Lou 2020 [A] {published data only}
Lou 2020 [B] {published data only}
-
- See first entry for publication details for this study. Lou 2020 [A].
Lynch 2021 {published data only}
MacMullan 2020 [A] {published data only}
MacMullan 2020 [B] {published data only}
-
- See first entry for publication details for this study. MacMullan 2020 [A].
MacMullan 2020 [C] {published data only}
-
- See first entry for publication details for this study. MacMullan 2020 [A].
MacMullan 2020 [D] {published data only}
-
- See first entry for publication details for this study. MacMullan 2020 [A].
Mairesse 2020 [A] {published data only}
Mairesse 2020 [B] {published data only}
-
- See first entry for publication details for this study. Mairesse 2020 [A].
Manalac 2020 [A] {published data only}
Manalac 2020 [B] {published data only}
-
- See first entry for publication details for this study. Manalac 2020 [A].
Marlet 2020 [A] {published data only}
-
- Marlet J, Petillon C, Ragot E, Abou El Fattah Y, Guillon A, Marchand Adam S, et al. Clinical performance of four immunoassays for antibodies to SARS-CoV-2, including a prospective analysis for the diagnosis of COVID-19 in a real-life routine care setting. Journal of Clinical Virology 2020;132:104633. - PMC - PubMed
Marlet 2020 [B] {published data only}
-
- See first entry for publication details for this study. Marlet 2020 [A].
Marlet 2020 [C] {published data only}
-
- See first entry for publication details for this study. Marlet 2020 [A].
Marlet 2020 [D] {published data only}
-
- See first entry for publication details for this study. Marlet 2020 [A].
Martinaud 2020 {published data only}
McAulay 2020 [A] {published data only}
McAulay 2020 [B] {published data only}
-
- See first entry for publication details for this study. McAulay 2020 [A].
McAulay 2020 [C] {published data only}
-
- See first entry for publication details for this study. McAulay 2020 [A].
McAulay 2020 [D] {published data only}
-
- See first entry for publication details for this study. McAulay 2020 [A].
Merrill 2020 [A] {published data only}
Merrill 2020 [B] {published data only}
-
- See first entry for publication details for this study. Merrill 2020 [A].
Montesinos 2020 [A] {published data only}
Montesinos 2020 [B] {published data only}
-
- See first entry for publication details for this study. Montesinos 2020 [A].
Montesinos 2020 [C] {published data only}
-
- See first entry for publication details for this study. Montesinos 2020 [A].
Montesinos 2020 [D] {published data only}
-
- See first entry for publication details for this study. Montesinos 2020 [A].
Montesinos 2020 [E] {published data only}
-
- See first entry for publication details for this study. Montesinos 2020 [A].
Montesinos 2020 [F] {published data only}
-
- See first entry for publication details for this study. Montesinos 2020 [A].
Montesinos 2020 [G] {published data only}
-
- See first entry for publication details for this study. Montesinos 2020 [A].
Muecksch 2020 [A] {published data only}
-
- Muecksch F, Wise H, Batchelor B, Squires M, Semple E, Richardson C, et al. Longitudinal analysis of clinical serology assay performance and neutralising antibody levels in COVID19 convalescents. medRxiv [Preprint] 2020. [DOI: ]
Muecksch 2020 [B] {published data only}
-
- See first entry for publication details for this study. Muecksch 2020 [A].
Muecksch 2020 [C] {published data only}
-
- See first entry for publication details for this study. Muecksch 2020 [A].
Muecksch 2020 [D] {published data only}
-
- See first entry for publication details for this study. Muecksch 2020 [A].
Naaber 2020 [A] {published data only}
Naaber 2020 [B] {published data only}
-
- See first entry for publication details for this study. Naaber 2020 [A].
Naaber 2020 [C] {published data only}
-
- See first entry for publication details for this study. Naaber 2020 [A].
Naaber 2020 [D] {published data only}
-
- See first entry for publication details for this study. Naaber 2020 [A].
Naaber 2020 [E] {published data only}
-
- See first entry for publication details for this study. Naaber 2020 [A].
Naaber 2020 [F] {published data only}
-
- See first entry for publication details for this study. Naaber 2020 [A].
Naaber 2020 [G] {published data only}
-
- See first entry for publication details for this study. Naaber 2020 [A].
Nagasawa 2020 [A] {published data only}
Nagasawa 2020 [B] {published data only}
-
- See first entry for publication details for this study. Nagasawa 2020 [A].
Nagasawa 2020 [C] {published data only}
-
- See first entry for publication details for this study. Nagasawa 2020 [A].
Nayak 2021 {published data only}
Ng 2020 [A] {published data only}
Ng 2020 [B] {published data only}
-
- See first entry for publication details for this study. Ng 2020 [A].
Nguyen 2020 {published data only}
Nicol 2020 [A] {published data only}
-
- Nicol T, Lefeuvre C, Serri O, Pivert A, Joubaud F, Dubee V, et al. Assessment of SARS-CoV-2 serological tests for the diagnosis of COVID-19 through the evaluation of three immunoassays: Two automated immunoassays (Euroimmun and Abbott) and one rapid lateral flow immunoassay (NG Biotech). Journal of Clinical Virology 2020;129:104511. - PMC - PubMed
Nicol 2020 [B] {published data only}
-
- See first entry for publication details for this study. Nicol 2020 [A].
Nicol 2020 [C] {published data only}
-
- See first entry for publication details for this study. Nicol 2020 [A].
Nicol 2020 [D] {published data only}
-
- See first entry for publication details for this study. Nicol 2020 [A].
Nicol 2020 [E] {published data only}
-
- See first entry for publication details for this study. Nicol 2020 [A].
Nilles 2020 [A] {published data only}
Nilles 2020 [B] {published data only}
-
- See first entry for publication details for this study. Nilles 2020 [A].
NSAE 2020 [A] {published data only}
-
- Public Health England. Evaluation of sensitivity and specificity of four commercially available SARS-CoV-2 antibody immunoassays (July 2020). https://assets.publishing.service.gov.uk/government/uploads/system/uploa....
NSAE 2020 [B] {published data only}
-
- See first entry for publication details for this study. NSAE 2020 [A].
NSAE 2020 [C] {published data only}
-
- See first entry for publication details for this study. NSAE 2020 [A].
NSAE 2020 [D] {published data only}
-
- See first entry for publication details for this study. NSAE 2020 [A].
Ong 2020 [A] {published data only}
Ong 2020 [B] {published data only}
-
- See first entry for publication details for this study. Ong 2020 [A].
Padoan 2020a {published data only}
-
- Padoan A, Cosma C, Sciacovelli L, Faggian D, Plebani M. Analytical performances of a chemiluminescence immunoassay for SARS-CoV-2 IgM/IgG and antibody kinetics. Clinical Chemistry and Laboratory Medicine 2020;58(7):1081-8. [DOI: ] - PubMed
Padoan 2020b [A] {published data only}
Padoan 2020b [B] {published data only}
-
- See first entry for publication details for this study. Padoan 2020b [A].
Paiva 2021 [A] {published data only}
-
- Paiva KJ, Grisson RD, Chan PA, Huard RC, Caliendo AM, Lonks JR, et al. Validation and performance comparison of three SARS-CoV-2 antibody assays. Journal of Medical Virology 2021;93(2):916-23. - PubMed
Paiva 2021 [B] {published data only}
-
- See first entry for publication details for this study. Paiva 2021 [A].
Paiva 2021 [C] {published data only}
-
- See first entry for publication details for this study. Paiva 2021 [A].
Pan 2020a {published data only}
Pape 2021 [A] {published data only}
-
- Pape C, Remme R, Wolny A, Olberg S, Wolf S, Cerrone L, et al. Microscopy-based assay for semi-quantitative detection of SARS-CoV-2 specific antibodies in human sera: A semi-quantitative, high throughput, microscopy-based assay expands existing approaches to measure SARS-CoV-2 specific antibody levels in human sera. Bioessays 2021;43(3):e2000257. - PMC - PubMed
Pape 2021 [B] {published data only}
-
- See first entry for publication details for this study. Pape 2021 [A].
Patel 2021 [A] {published data only}
Patel 2021 [B] {published data only}
-
- See first entry for publication details for this study. Patel 2021 [A].
Patel 2021 [C] {published data only}
-
- See first entry for publication details for this study. Patel 2021 [A].
Patel 2021 [D] {published data only}
-
- See first entry for publication details for this study. Patel 2021 [A].
Patel 2021 [E] {published data only}
-
- See first entry for publication details for this study. Patel 2021 [A].
Pere 2020 {published data only}
Perez‐Garcia 2020(a) {published data only}
-
- Pérez-García F, Pérez-Tanoira R, Romanyk J, Arroyo T, Gómez-Herruz P, Cuadros-González J. Rapid diagnosis of SARS-CoV-2 infection by detecting IgG and IgM antibodies with an immunochromatographic device: a prospective single-center study. medRxiv [Preprint] 2020. [DOI: ]
-
- Perez-Garcia F, Perez-Tanoira R, Romanyk J, Arroyo T, Gomez-Herruz P, Cuadros-Gonzalez J. Alltest rapid lateral flow immunoassays is reliable in diagnosing SARS-CoV-2 infection from 14 days after symptom onset: A prospective single-center study. Journal of Clinical Virology 2020;129:104473. - PMC - PubMed
Perez‐Garcia 2020(b) {published data only}
-
- Pérez-García F, Pérez-Tanoira R, Romanyk J, Arroyo T, Gómez-Herruz P, Cuadros-González J. Rapid diagnosis of SARS-CoV-2 infection by detecting IgG and IgM antibodies with an immunochromatographic device: a prospective single-center study. medRxiv [Preprint] 2020. [DOI: ]
-
- Perez-Garcia F, Perez-Tanoira R, Romanyk J, Arroyo T, Gomez-Herruz P, Cuadros-Gonzalez J. Alltest rapid lateral flow immunoassays is reliable in diagnosing SARS-CoV-2 infection from 14 days after symptom onset: A prospective single-center study. Journal of Clinical Virology 2020;129:104473. - PMC - PubMed
Perez‐Garcia 2021 [A] {published data only}
Perez‐Garcia 2021 [B] {published data only}
-
- See first entry for publication details for this study. Perez-Garcia 2021 [A].
Perez‐Garcia 2021 [C] {published data only}
-
- See first entry for publication details for this study. Perez-Garcia 2021 [A].
Perez‐Garcia 2021 [D] {published data only}
-
- See first entry for publication details for this study. Perez-Garcia 2021 [A].
Perez‐Garcia 2021 [E] {published data only}
-
- See first entry for publication details for this study. Perez-Garcia 2021 [A].
Perez‐Garcia 2021 [F] {published data only}
-
- See first entry for publication details for this study. Perez-Garcia 2021 [A].
Pfluger 2020 [A] {published data only}
Pfluger 2020 [B] {published data only}
-
- See first entry for publication details for this study. Pfluger 2020 [A].
Pfluger 2020 [C] {published data only}
-
- See first entry for publication details for this study. Pfluger 2020 [A].
Pfluger 2020 [D] {published data only}
-
- See first entry for publication details for this study. Pfluger 2020 [A].
Pfluger 2020 [E] {published data only}
-
- See first entry for publication details for this study. Pfluger 2020 [A].
PHE 2020 [A] {published data only}
-
- Public Health England. Evaluation of DiaSorin LIAISON SARSCoV-2 S1/S2 IgG serology assay for the detection of anti-SARS-CoV-2 antibodies. https://assets.publishing.service.gov.uk/government/uploads/system/uploa....
-
- Public Health England. Evaluation of Roche Elecsys Anti-SARS-CoV-2 serology assay for the detection of anti-SARS-CoV-2 antibodies. https://assets.publishing.service.gov.uk/government/uploads/system/uploa....
-
- Public Health England. Evaluation of Siemens Atellica-IM Total (COV2T) SARS-CoV-2 serology assay for the detection of anti-SARS-CoV-2 total antibodies. https://assets.publishing.service.gov.uk/government/uploads/system/uploa....
-
- Public Health England. Evaluation of the Abbott SARS-CoV-2 IgG for the detection of anti-SARS-CoV-2 antibodies. https://assets.publishing.service.gov.uk/government/uploads/system/uploa....
-
- Public Health England. Evaluation of the Beckman Coulter Access Anti-SARS-CoV-2 IgG assay for the detection of anti-SARS-CoV-2 antibodies. https://assets.publishing.service.gov.uk/government/uploads/system/uploa....
PHE 2020 [B] {published data only}
-
- See first entry for publication details for this study. PHE 2020 [A].
PHE 2020 [C] {published data only}
-
- See first entry for publication details for this study. PHE 2020 [A].
PHE 2020 [D] {published data only}
-
- See first entry for publication details for this study. PHE 2020 [A].
PHE 2020 [E] {published data only}
-
- See first entry for publication details for this study. PHE 2020 [A].
PHE 2020 [F] {published data only}
-
- See first entry for publication details for this study. PHE 2020 [A].
PHE 2020 [G] {published data only}
-
- See first entry for publication details for this study. PHE 2020 [A].
PHE 2020 [H] {published data only}
-
- See first entry for publication details for this study. PHE 2020 [A].
Phipps 2020 {published data only}
Pickering 2020 [A] {published data only}
-
- Pickering S, Betancor G, Galao RP, Merrick B, Signell AW, Wilson HD, et al. Comparative assessment of multiple COVID-19 serological technologies supports continued evaluation of point-of-care lateral flow assays in hospital and community healthcare settings. PLoS Pathogens 2020;16(9):e1008817. - PMC - PubMed
-
- Pickering S, Betancor G, Pedro Galao R, Merrick B, Signell AW, Wilson HD, et al. Comparative assessment of multiple COVID-19 serological technologies supports continued evaluation of point-of-care lateral flow assays in hospital and community healthcare settings. medRxiv [Preprint] 2020. [DOI: ] - PMC - PubMed
Pickering 2020 [B] {published data only}
-
- See first entry for publication details for this study. Pickering 2020 [A].
Pickering 2020 [C] {published data only}
-
- See first entry for publication details for this study. Pickering 2020 [A].
Pickering 2020 [D] {published data only}
-
- See first entry for publication details for this study. Pickering 2020 [A].
Pickering 2020 [E] {published data only}
-
- See first entry for publication details for this study. Pickering 2020 [A].
Pickering 2020 [F] {published data only}
-
- See first entry for publication details for this study. Pickering 2020 [A].
Pickering 2020 [G] {published data only}
-
- See first entry for publication details for this study. Pickering 2020 [A].
Pickering 2020 [H] {published data only}
-
- See first entry for publication details for this study. Pickering 2020 [A].
Pickering 2020 [I] {published data only}
-
- See first entry for publication details for this study. Pickering 2020 [A].
Pickering 2020 [J] {published data only}
-
- See first entry for publication details for this study. Pickering 2020 [A].
Pollan 2020 {published data only}
Prazuck 2020 [A] {published data only}
Prazuck 2020 [B] {published data only}
-
- See first entry for publication details for this study. Prazuck 2020 [A].
Qian 2020a {published data only}
-
- Qian C, Zhou M, Cheng F, Lin X, Gong Y, Xie X, et al. Development and multicenter performance evaluation of fully automated SARS-CoV-2 IgM and IgG immunoassays. Clinical Chemistry and Laboratory Medicine 2020;58(9):1601-7. - PubMed
Qiu 2020 {published data only}
Ragnesola 2020 {published data only}
Renard 2021 [A] {published data only}
-
- Renard N, Daniel S, Cayet N, Pecquet M, Raymond F, Pons S, et al. Performance characteristics of the VIDAS® SARS-COV-2 IgM and IgG serological assays. medRxiv [Preprint] 2020. [https://doi.org/10.1101/2020.09.28.20196030] - PMC - PubMed
Renard 2021 [B] {published data only}
-
- See first entry for publication details for this study. Renard 2020 [A].
Rijkers 2020 {published data only}
Rode 2021 [A] {published data only}
-
- Rode OD, Kurolt IC, Puljiz I, Civljak R, Balent NC, Laskaj R, et al. Antibody response and the clinical presentation of patients with COVID-19 in Croatia: the importance of a two-step testing approach. European Journal of Clinical Microbiology and Infectious Diseases 2021;40(2):261-8. - PMC - PubMed
Rode 2021 [B] {published data only}
-
- See first entry for publication details for this study. Rode 2021 [A].
Rode 2021 [C] {published data only}
-
- See first entry for publication details for this study. Rode 2021 [A].
Rudolf 2020 [A] {published data only}
-
- Rudolf F, Kaltenbach H-M, Linnik J, Ruf M-T, Niederhauser C, Nickel B, et al. Clinical characterisation of eleven lateral flow assays for detection of COVID-19 antibodies in a population. medRxiv [Preprint] 2020. [DOI: ]
Rudolf 2020 [B] {published data only}
-
- See first entry for publication details for this study. Rudolf 2020 [A].
Rudolf 2020 [C] {published data only}
-
- See first entry for publication details for this study. Rudolf 2020 [A].
Rudolf 2020 [D] {published data only}
-
- See first entry for publication details for this study. Rudolf 2020 [A].
Rudolf 2020 [E] {published data only}
-
- See first entry for publication details for this study. Rudolf 2020 [A].
Rudolf 2020 [F] {published data only}
-
- See first entry for publication details for this study. Rudolf 2020 [A].
Rudolf 2020 [G] {published data only}
-
- See first entry for publication details for this study. Rudolf 2020 [A].
Rudolf 2020 [H] {published data only}
-
- See first entry for publication details for this study. Rudolf 2020 [A].
Rudolf 2020 [I] {published data only}
-
- See first entry for publication details for this study. Rudolf 2020 [A].
Rudolf 2020 [J] {published data only}
-
- See first entry for publication details for this study. Rudolf 2020 [A].
Rudolf 2020 [K] {published data only}
-
- See first entry for publication details for this study. Rudolf 2020 [A].
Ruetalo 2020 [A] {published data only}
-
- Ruetalo N, Businger R, Althaus K, Fink S, Ruoff F, Hamprecht K, et al. Neutralizing antibody response in non-hospitalized SARS-CoV-2 patients. medRxiv [Preprint] 2020. [DOI: ]
Ruetalo 2020 [B] {published data only}
-
- See first entry for publication details for this study. Ruetalo 2020 [A].
Ruetalo 2020 [C] {published data only}
-
- See first entry for publication details for this study. Ruetalo 2020 [A].
Schnurra 2020 [A] {published data only}
Schnurra 2020 [B] {published data only}
-
- See first entry for publication details for this study. Schnurra 2020 [A].
Schnurra 2020 [C] {published data only}
-
- See first entry for publication details for this study. Schnurra 2020 [A].
Schnurra 2020 [D] {published data only}
-
- See first entry for publication details for this study. Schnurra 2020 [A].
Schnurra 2020 [E] {published data only}
-
- See first entry for publication details for this study. Schnurra 2020 [A].
Schnurra 2020 [F] {published data only}
-
- See first entry for publication details for this study. Schnurra 2020 [A].
Schnurra 2020 [G] {published data only}
-
- See first entry for publication details for this study. Schnurra 2020 [A].
Serre‐Miranda 2021 [A] {published data only}
Serre‐Miranda 2021 [B] {published data only}
-
- See first entry for publication details for this study. Serre-Miranda 2021 [A].
Serre‐Miranda 2021 [C] {published data only}
-
- See first entry for publication details for this study. Serre-Miranda 2021 [A].
Serre‐Miranda 2021 [D] {published data only}
-
- See first entry for publication details for this study. Serre-Miranda 2021 [A].
Serre‐Miranda 2021 [E] {published data only}
-
- See first entry for publication details for this study. Serre-Miranda 2021 [A].
Serre‐Miranda 2021 [F] {published data only}
-
- See first entry for publication details for this study. Serre-Miranda 2021 [A].
Serre‐Miranda 2021 [G] {published data only}
-
- See first entry for publication details for this study. Serre-Miranda 2021 [A].
Serre‐Miranda 2021 [H] {published data only}
-
- See first entry for publication details for this study. Serre-Miranda 2021 [A].
Serre‐Miranda 2021 [I] {published data only}
-
- See first entry for publication details for this study. Serre-Miranda 2021 [A].
Serre‐Miranda 2021 [J] {published data only}
-
- See first entry for publication details for this study. Serre-Miranda 2021 [A].
Serre‐Miranda 2021 [K] {published data only}
-
- See first entry for publication details for this study. Serre-Miranda 2021 [A].
Serre‐Miranda 2021 [L] {published data only}
-
- See first entry for publication details for this study. Serre-Miranda 2021 [A].
Shamsollahi 2020 {published data only}
-
- Shamsollahi HR, Amini M, Alizadeh S, Nedjat S, Akbari-Sari A, Rezaei M, et al. Assessment of a serological diagnostic kit of sars-cov-2 available in Iran. medRxiv [Preprint] 2020. [DOI: ] - PubMed
-
- Shamsollahi HR, Amini M, Alizadeh S, Nedjat S, Akbari-Sari A, Rezaei M, et al. Validity of a serological diagnostic kit of SARS-CoV-2 available in Iran. Archives of Iranian Medicine 2020;23(9):629-32. - PubMed
Shen 2020a {published data only}
Shen 2020b {published data only}
Soleimani 2021 {published data only}
Sterlin 2021 [A] {published data only}
Sterlin 2021 [B] {published data only}
-
- See first entry for publication details for this study. Sterlin 2021 [A].
Suhandynata 2020a {published data only}
Suhandynata 2020b [A] {published data only}
Suhandynata 2020b [B] {published data only}
-
- See first entry for publication details for this study. Suhandynata 2020b [A].
Suhandynata 2020b [C] {published data only}
-
- See first entry for publication details for this study. Suhandynata 2020b [A].
Sun 2020 {published data only}
Sweeney 2020 {published data only}
-
- Sweeney N, Merrick B, Galão RP, Pickering S, Botros A, Wilson H, et al. Clinical utility of targeted SARS-CoV-2 serology testing to aid the diagnosis and management of suspected missed, late or post-COVID-19 infection syndromes: results from a pilot service. medRxiv [Preprint] 2020. [DOI: ] - PMC - PubMed
Tan 2020 [A] {published data only}
-
- Tan SS, Saw S, Chew KL, Wang C, Pajarillaga A, Khoo C, et al. Comparative clinical evaluation of the Roche Elecsys and Abbott SARS-CoV-2 serology assays for COVID-19. Archives of Pathology and Laboratory Medicine 2020;145:32-8. - PubMed
Tan 2020 [B] {published data only}
-
- See first entry for publication details for this study. Tan 2020 [A].
Tang 2020 [A] {published data only}
Tang 2020 [B] {published data only}
-
- See first entry for publication details for this study. Tang 2020 [A].
Tang 2020 [C] {published data only}
-
- See first entry for publication details for this study. Tang 2020 [A].
Theel 2020 [A] {published data only}
Theel 2020 [B] {published data only}
-
- See first entry for publication details for this study. Theel 2020 [A].
Theel 2020 [C] {published data only}
-
- See first entry for publication details for this study. Theel 2020 [A].
Theel 2020 [D] {published data only}
-
- See first entry for publication details for this study. Theel 2020 [A].
Thijsen 2020 {published data only}
Trabaud 2020 [A] {published data only}
Trabaud 2020 [B] {published data only}
-
- See first entry for publication details for this study. Trabaud 2020 [A].
Trabaud 2020 [C] {published data only}
-
- See first entry for publication details for this study. Trabaud 2020 [A].
Trabaud 2020 [D] {published data only}
-
- See first entry for publication details for this study. Trabaud 2020 [A].
Trabaud 2020 [E] {published data only}
-
- See first entry for publication details for this study. Trabaud 2020 [A].
Trabaud 2020 [F] {published data only}
-
- See first entry for publication details for this study. Trabaud 2020 [A].
Trabaud 2020 [G] {published data only}
-
- See first entry for publication details for this study. Trabaud 2020 [A].
Trabaud 2020 [H] {published data only}
-
- See first entry for publication details for this study. Trabaud 2020 [A].
Traugott 2020 [A] {published data only}
-
- Traugott M, Aberle SW, Aberle JH, Griebler H, Karolyi M, Pawelka E, et al. Performance of severe acute respiratory syndrome coronavirus 2 antibody assays in different stages of infection: comparison of commercial enzyme-linked immunosorbent assays and rapid Tests. Journal of Infectious Diseases 2020;222(3):362-6. - PMC - PubMed
Traugott 2020 [B] {published data only}
-
- See first entry for publication details for this study. Tragoutt 2020 [A].
Traugott 2020 [C] {published data only}
-
- See first entry for publication details for this study. Tragoutt 2020 [A].
Traugott 2020 [D] {published data only}
-
- See first entry for publication details for this study. Tragoutt 2020 [A].
Traugott 2020 [E] {published data only}
-
- See first entry for publication details for this study. Tragoutt 2020 [A].
Traugott 2020 [F] {published data only}
-
- See first entry for publication details for this study. Tragoutt 2020 [A].
Tre‐Hardy 2021 [A] {published data only}
Tre‐Hardy 2021 [B] {published data only}
-
- See first entry for publication details for this study. Tre-Hardy 2021 [A].
Tuaillon 2020 [A] {published data only}
Tuaillon 2020 [B] {published data only}
-
- See first entry for publication details for this study. Tuallion 2020 [A].
Tuaillon 2020 [C] {published data only}
-
- See first entry for publication details for this study. Tuallion 2020 [A].
Tuaillon 2020 [D] {published data only}
-
- See first entry for publication details for this study. Tuallion 2020 [A].
Tuaillon 2020 [E] {published data only}
-
- See first entry for publication details for this study. Tuallion 2020 [A].
Tuaillon 2020 [F] {published data only}
-
- See first entry for publication details for this study. Tuallion 2020 [A].
Tuaillon 2020 [G] {published data only}
-
- See first entry for publication details for this study. Tuallion 2020 [A].
Tuaillon 2020 [H] {published data only}
-
- See first entry for publication details for this study. Tuallion 2020 [A].
Tuaillon 2020 [I] {published data only}
-
- See first entry for publication details for this study. Tuallion 2020 [A].
Tuaillon 2020 [J] {published data only}
-
- See first entry for publication details for this study. Tuallion 2020 [A].
Valdivia 2020 [A] {published data only}
-
- Valdivia A, Torres I, Latorre V, Francés-Gómez C, Albert E, Gozalbo-Rovira R, et al. Inference of SARS-CoV-2 spike-binding neutralizing antibody titers in sera from hospitalized COVID-19 patients by using commercial enzyme and chemiluminescent immunoassays. medRxiv [Preprint] 2020. [DOI: ] - PMC - PubMed
Valdivia 2020 [B] {published data only}
-
- See first entry for publication details for this study. Valdivia 2020 [A].
Valdivia 2020 [C] {published data only}
-
- See first entry for publication details for this study. Valdivia 2020 [A].
Valdivia 2020 [D] {published data only}
-
- See first entry for publication details for this study. Valdivia 2020 [A].
Van Elslande 2020a [A] {published data only}
Van Elslande 2020a [B] {published data only}
-
- See first entry for publication details for this study. Van Elslande 2020a [A].
Van Elslande 2020a [C] {published data only}
-
- See first entry for publication details for this study. Van Elslande 2020a [A].
Van Elslande 2020a [D] {published data only}
-
- See first entry for publication details for this study. Van Elslande 2020a [A].
Van Elslande 2020a [E] {published data only}
-
- See first entry for publication details for this study. Van Elslande 2020a [A].
Van Elslande 2020a [F] {published data only}
-
- See first entry for publication details for this study. Van Elslande 2020a [A].
Van Elslande 2020a [G] {published data only}
-
- See first entry for publication details for this study. Van Elslande 2020a [A].
Van Elslande 2020a [H] {published data only}
-
- See first entry for publication details for this study. Van Elslande 2020a [A].
Van Elslande 2020b [A] {published data only}
Van Elslande 2020b [B] {published data only}
-
- See first entry for publication details for this study. Van Elslande 2020b [A].
Van Elslande 2020b [C] {published data only}
-
- See first entry for publication details for this study. Van Elslande 2020b [A].
Van Elslande 2020b [D] {published data only}
-
- See first entry for publication details for this study. Van Elslande 2020b [A].
Van Elslande 2020b [E] {published data only}
-
- See first entry for publication details for this study. Van Elslande 2020b [A].
Van Elslande 2020b [F] {published data only}
-
- See first entry for publication details for this study. Van Elslande 2020b [A].
Van Elslande 2020b [G] {published data only}
-
- See first entry for publication details for this study. Van Elslande 2020b [A].
Velay 2020 [A] {published data only}
Velay 2020 [B] {published data only}
-
- See first entry for publication details for this study. Velay 2020 [A].
Velay 2020 [C] {published data only}
-
- See first entry for publication details for this study. Velay 2020 [A].
Velay 2020 [D] {published data only}
-
- See first entry for publication details for this study. Velay 2020 [A].
Veyrenche 2021 [A] {published data only}
Veyrenche 2021 [B] {published data only}
-
- See first entry for publication details for this study. Veyrenche 2021 [A].
Veyrenche 2021 [C] {published data only}
-
- See first entry for publication details for this study. Veyrenche 2021 [A].
Veyrenche 2021 [D] {published data only}
-
- See first entry for publication details for this study. Veyrenche 2021 [A].
Wang 2020a {published data only}
Weidner 2020 [A] {published data only}
Weidner 2020 [B] {published data only}
-
- See first entry for publication details for this study. Weidner 2020 [A].
Weidner 2020 [C] {published data only}
-
- See first entry for publication details for this study. Weidner 2020 [A].
Weidner 2020 [D] {published data only}
-
- See first entry for publication details for this study. Weidner 2020 [A].
Weidner 2020 [E] {published data only}
-
- See first entry for publication details for this study. Weidner 2020 [A].
Weidner 2020 [F] {published data only}
-
- See first entry for publication details for this study. Weidner 2020 [A].
Wellinghausen 2020a [A] {published data only}
Wellinghausen 2020a [B] {published data only}
-
- See first entry for publication details for this study. Wellinghausen 2020a [A].
Wellinghausen 2020a [C] {published data only}
-
- See first entry for publication details for this study. Wellinghausen 2020a [A].
Wellinghausen 2020a [D] {published data only}
-
- See first entry for publication details for this study. Wellinghausen 2020a [A].
Wellinghausen 2020a [E] {published data only}
-
- See first entry for publication details for this study. Wellinghausen 2020a [A].
Wellinghausen 2020b {published data only}
Whitman 2020a [A] {published data only}
Whitman 2020a [B] {published data only}
-
- See first entry for publication details for this study. Whitman 2020a [A].
Whitman 2020a [C] {published data only}
-
- See first entry for publication details for this study. Whitman 2020a [A].
Whitman 2020a [D] {published data only}
-
- See first entry for publication details for this study. Whitman 2020a [A].
Whitman 2020a [E] {published data only}
-
- See first entry for publication details for this study. Whitman 2020a [A].
Whitman 2020a [F] {published data only}
-
- See first entry for publication details for this study. Whitman 2020a [A].
Whitman 2020a [G] {published data only}
-
- See first entry for publication details for this study. Whitman 2020a [A].
Whitman 2020a [H] {published data only}
-
- See first entry for publication details for this study. Whitman 2020a [A].
Whitman 2020a [I] {published data only}
-
- See first entry for publication details for this study. Whitman 2020a [A].
Whitman 2020a [J] {published data only}
-
- See first entry for publication details for this study. Whitman 2020a [A].
Whitman 2020a [K] {published data only}
-
- See first entry for publication details for this study. Whitman 2020a [A].
Whitman 2020b [A] {published data only}
-
- Whitman JD, Hiatt J, Mowery CT, Shy BR, Yu R, Yamamoto TN, et al. Test performance evaluation of SARS-CoV-2 serological assays. medRxiv [Preprint] 2020. [DOI: 10.1101/2020.04.25.20074856] - DOI
Whitman 2020b [B] {published data only}
-
- See first entry for publication details for this study. Whitman 2020b [A].
Whitman 2020b [C] {published data only}
-
- See first entry for publication details for this study. Whitman 2020b [A].
Wolff 2020 [A] {published data only}
Wolff 2020 [B] {published data only}
-
- See first entry for publication details for this study. Wolff 2020 [A].
Wolff 2020 [C] {published data only}
-
- See first entry for publication details for this study. Wolff 2020 [A].
Wolff 2020 [D] {published data only}
-
- See first entry for publication details for this study. Wolff 2020 [A].
Wolff 2020 [E] {published data only}
-
- See first entry for publication details for this study. Wolff 2020 [A].
Wolff 2020 [F] {published data only}
-
- See first entry for publication details for this study. Wolff 2020 [A].
Wu 2020 [A] {published data only}
Wu 2020 [B] {published data only}
-
- See first entry for publication details for this study. Wu 2020 [A].
Wu 2020 [C] {published data only}
-
- See first entry for publication details for this study. Wu 2020 [A].
Wu 2020 [D] {published data only}
-
- See first entry for publication details for this study. Wu 2020 [A].
Xiang 2020a {published data only}
Xiao 2020a {published data only}
Xiao 2020b [A] {published data only}
Xiao 2020b [B] {published data only}
-
- See first entry for publication details for this study. Xiao 2020b [A].
Xiao 2020b [C] {published data only}
-
- See first entry for publication details for this study. Xiao 2020b [A].
Xiao 2020b [D] {published data only}
-
- See first entry for publication details for this study. Xiao 2020b [A].
Yang 2020 [A] {published data only}
Yang 2020 [B] {published data only}
-
- See first entry for publication details for this study. Yang 2020 [A].
Yongchen 2020 {published data only}
Zhang 2020a [A] {published data only}
Zhang 2020a [B] {published data only}
-
- See first entry for publication details for this study. Zhang 2020a [A].
Zhang 2020b [A] {published data only}
Zhang 2020b [B] {published data only}
-
- See first entry for publication details for this study. Zhang 2020b [A].
Zhang 2020b [C] {published data only}
-
- See first entry for publication details for this study. Zhang 2020b [A].
Zhao 2020a [A] {published data only}
Zhao 2020a [B] {published data only}
-
- See first entry for publication details for this study. Zhao 2020a [A].
Zhao 2020a [C] {published data only}
-
- See first entry for publication details for this study. Zhao 2020a [A].
References to studies excluded from this review
Abravanel 2020 {published data only}
Adams 2020b {published data only}
-
- Adams ER, Anand R, Andersson MI, Auckland K, Baillie JK, Barnes E, et al. Evaluation of antibody testing for SARS-Cov-2 using ELISA and lateral flow immunoassays. medRxiv [Preprint] 2020. [www.medrxiv.org/content/10.1101/2020.04.15.20066407v1.full.pdf]
Alger 2020 {published data only}
Amanat 2020 {published data only}
Amrun 2020 {published data only}
Antoine‐Reid 2020 {published data only}
Arumugam 2020 {published data only}
-
- Arumugam A, Wong SS. The potential use of unprocessed sample for RT-qPCR detection of COVID-19 without an RNA extraction step. bioRxiv [Preprint] 2020. [DOI: 10.1101/2020.04.06.028811] - DOI
Ayouba 2020 {published data only}
Barallat 2020 {published data only}
-
- Barallat J, Fernández-Rivas G, Quirant-Sánchez B, González V, Doladé M, Martinez-Caceres E, et al. Seroprevalence of SARS-CoV-2 IgG Specific Antibodies among Healthcare Workers in the Northern Metropolitan Area of Barcelona, Spain, after the first pandemic wave. medRxiv [Preprint] 2020. [DOI: ] - PMC - PubMed
Baron 2020 {published data only}
-
- Baron RC, Risch L, Weber M, Thiel S, Grossmann K, Wohlwend N, et al. Frequency of serological non-responders and false-negative RT-PCR results in SARS-CoV-2 testing: a population-based study. Clinical Chemistry and Laboratory Medicine 2020;58(12):2131-40. - PubMed
Batra 2020 {published data only}
Becker 2020 {published data only}
-
- Becker M, Strengert M, Junker D, Kerrinnes T, Kaiser PD, Traenkle B, et al. Going beyond clinical routine in SARS-CoV-2 antibody testing - A multiplex corona virus antibody test for the evaluation of cross-reactivity to endemic coronavirus antigens. medRxiv [Preprint] 2020. [DOI: ]
Bendavid 2020 {published data only}
Black 2020 {published data only}
Bortz 2020 {published data only}
Brandstetter 2020 {published data only}
-
- Brandstetter S, Roth S, Harner S, Buntrock-Dopke H, Toncheva AA, Borchers N, et al. Symptoms and immunoglobulin development in hospital staff exposed to a SARS-CoV-2 outbreak. Pediatric Allergy and Immunology 2020;31(7):841-7. - PubMed
Brantley 2020 {published data only}
-
- Brantley HL, Yoo RM, Jones GI, Stock MA, Park PJ, Sheils NE, et al. Variation across population subgroups of COVID-19 antibody testing performance. medRxiv [Preprint] 2020. [DOI: ]
Brecher 2020 {published data only}
Bruni 2020 {published data only}
Bryan 2020b {published data only}
Buntinx 2020 {published data only}
-
- Buntinx F, Claes P, Gulikers M, Verbakel JY, De Lepeleire J, Van der Elst M, et al. Early experiences with antibody testing in a Flemish nursing home during an acute COVID-19 outbreak: a retrospective cohort study. medRxiv [Preprint] 2020. [DOI: ]
Burbelo 2020 {published data only}
-
- Burbelo PD, Riedo FX, Morishima C, Rawlings S, Smith D, Das S, et al. Detection of nucleocapsid antibody to SARS-CoV-2 is more sensitive than antibody to spike protein in COVID-19 patients. MedRxiv [Preprint] 2020. [DOI: ]
Byrnes 2020 {published data only}
Cai 2020 {published data only}
Cassaniti 2020 {published data only}
-
- Cassaniti I, Novazzi F, Giardina F, Salivaro F, Sachs M, Perlini S, et al. Performance of VivaDiagTM COVID-19 IgM/IgG Rapid Test is inadequate for diagnosis of COVID-19 in acute patients referring to emergency room department. Journal of Medical Virology 2020;92(10):1724-7. [DOI: 10.1002/jmv.25800] - DOI - PMC - PubMed
Chatzidimitriou 2020 {published data only}
Chen 2020a {published data only}
-
- Chen Z, Zhang Z, Zhai X, Li Y, Lin L, Zhao H, et al. Rapid and sensitive detection of anti-SARS-CoV-2 IgG using lanthanide-doped nanoparticles-based lateral flow immunoassay. Analytical Chemistry 2020;92(10):7226-31. - PubMed
Choe 2020 {published data only}
Chughtai 2020 {published data only}
-
- Chughtai OR, Batool H, Khan MD, Ashraf S. Seroconversion in newly diagnosed cases of coronavirus disease. Journal of the College of Physicians and Surgeons--Pakistan 2020;30(8):801-4. - PubMed
Colavita 2020 {published data only}
-
- Colavita F, Brogi A, Lapa D, Bordi L, Matusali G, Meschi S, et al. Evaluation of ELISA tests for the qualitative determination of IgG, IgM and IgA to SARS-CoV-2. medRxiv [Preprint] 2020. [DOI: ]
Comar 2020 {published data only}
-
- Comar M, Brumat M, Concas MP, Argentini G, Bianco A, Bicego L, et al. COVID-19 experience: first Italian survey on healthcare staff members from a mother-child research hospital using combined molecular and rapid immunoassays test. MedRxiv [Preprint] 2020. [DOI: 10.1101/2020.04.19.20071563] - DOI
Dahlke 2020 {published data only}
-
- Dahlke C, Heidepriem J, Kobbe R, Santer R, Koch T, Fathi A. Distinct early IgA profile may determine severity of COVID-19 symptoms: an immunological case series. medRxiv [Preprint] 2020. [DOI: 10.1101/2020.04.14.20059733] - DOI
Das 2020 {published data only}
Demey 2020 {published data only}
Di Lorenzo 2020 {published data only}
-
- Di Lorenzo G, Toniolo P, Lurani C, Foresti L, Carrisi C. Evaluating the adequacy of Prima Covid-19 IgG/IgM Rapid Test for the assessment of exposure to SARS-CoV-2 virus. medRxiv [Preprint] 2020. [DOI: ]
Dittadi 2020 {published data only}
-
- Dittadi R, Afshar H, Carraro P. The early antibody response to SARS-Cov-2 infection. Clinical Chemistry and Laboratory Medicine 2020;58(10):e201-3. - PubMed
Dobaño 2020 {published data only}
Dohla 2020 {published data only}
Du 2020 {published data only}
Edouard 2020 {published data only}
Erikstrup 2020 {published data only}
-
- Erikstrup C, Hother CE, Vestager Pedersen OB, Mølbak K, Skov RL, Holm DK, et al. Estimation of SARS-CoV-2 infection fatality rate by real-time antibody screening of blood donors. medRxiv [Preprint] 2020. [https://doi.org/10.1101/2020.04.24.20075291] - PMC - PubMed
Espino 2020 {published data only}
-
- Espino AM, Pantoja P, Sariol CA. Validation and performance of a quantitative IgG assay for the screening of SARS-CoV-2 antibodies. bioRxiv [Preprint] 2020. [DOI: ]
Fong 2020 {published data only}
Freeman 2020 {published data only}
-
- Freeman B, Lester S, Mills L, Rasheed MA, Moye S, Abiona O, et al. Validation of a SARS-CoV-2 spike ELISA for use in contact investigations and serosurveillance. bioRxiv [Preprint] 2020. [DOI: ]
Garcia‐Basteiro 2020 {published data only}
Garcia Garmendia 2020 {published data only}
-
- Garcia Garmendia JL, Ramirez Arcos M, Barrero Almodovar AE, Chavez Caballero M, Jorge Amigo V, Serrano Martino MC. Viral detection and serological response in critically ill patients with SARS-CoV-2. Implications for isolation withdrawal [Detección viral y respuesta serológica en pacientes críticos intubados con SARS-CoV-2. Implicaciones para retirada de aislamiento]. Medicina Intensiva 2020;44(9):586-8. - PMC - PubMed
Grzelak 2020 {published data only}
-
- Grzelak L, Temmam S, Planchais C, Demeret C, Huon C, Guivel F, et al. SARS-CoV-2 serological analysis of COVID-19 hospitalized patients, pauci-symptomatic individuals and blood donors. medRxiv [Preprint] 2020. [DOI: ]
Guo 2020a {published data only}
Guo 2020c {published data only}
-
- Guo X, Guo Z, Duan C, Chen Z, Wang G, Lu Y, et al. Long- term persistence of IgG antibodies in SARS-CoV infected healthcare workers. medRxiv [Preprint] 2020. [DOI: 10.1101/2020.02.12.20021386] - DOI
Guthmiller 2020 {published data only}
He 2020 {published data only}
He 2020a {published data only}
Hou 2020 {published data only}
Huang 2020a {published data only}
Huang 2020b {published data only}
Huang 2020c {published data only}
-
- Huang J, Mao T, Li S, Wu L, Xu X, Li H, et al. Long period dynamics of viral load and antibodies for SARS-CoV-2 infection: an observational cohort study. medRxiv [Preprint] 2020. [DOI: ]
Hung 2020 {published data only}
Imam 2020 {published data only}
-
- Imam M, Khawaja S, Naz A, Siddiqui A, Nafees TS, Younus A, et al. Performance assessment of first-generation anti-SARS-CoV-2 serological assays. medRxiv [Preprint] 2020. [DOI: ]
Infantino 2020 {published data only}
Jia 2020 {published data only}
Jiang 2020b {published data only}
Karp 2020 {published data only}
Karp 2020a {published data only}
Klumpp‐Thomas 2020 {published data only}
Kruttgen 2020 {published data only}
Kushemererwa 2020 {published data only}
-
- Kushemererwa GE, Kayongo I, Semanda P, Nansumba H, Tadeo I, Namulindwa C, et al. Combination of antibody based rapid diagnostic tests used in an algorithm may improve their performance in SARS CoV-2 diagnosis. medRxiv [Preprint] 2020. [DOI: ]
Lahner 2020 {published data only}
-
- Lahner E, Dilaghi E, Prestigiacomo C, Alessio G, Marcellini L, Simmaco M, et al. Prevalence of SARS-Cov-2 infection in health workers (HWs) and diagnostic test performance: the experience of a teaching hospital in central Italy. International Journal of Environmental Research and Public Health 2020;17(12):4417. - PMC - PubMed
Lapuente 2020 {published data only}
Lee 2020 {published data only}
Lei 2020 {published data only}
-
- Lei Q, Li Y, Hou H, Wang F, Ouyang Z, Zhang Y, et al. Antibody dynamics to SARS-CoV-2 in asymptomatic and mild COVID-19 patients. medRxiv [Preprint] 2020. [DOI: ]
Li 2020a {published data only}
Li 2020b {published data only}
Li 2020c {published data only}
Li 2020d {published data only}
-
- Li Y, He Q, Yu R, Jiang H, Wang W, Feng D, et al. Highlighted prospects of an IgM/IgG antibodies test in identifying individuals with asymptomatic SARS CoV-2 infection. Archives of Pathology and Laboratory Medicine 2020;145:39-45. - PubMed
Li 2020e {published data only}
Lin 2020 {published data only}
Linares 2020 {published data only}
-
- Linares CA, Ryan F, Moses SE. Early data on the performance of a combined SARS-CoV-2 spike-nucleocapsid antibody lateral flow device compared to a nucleocapsid-only device. bioRxiv [Preprint] 2020. [DOI: ] - PubMed
Lippi 2020 {published data only}
-
- Lippi G, Salvagno GL, Pegoraro M, Militello V, Caloi C, Peretti A, et al. Preliminary evaluation of Roche Cobas Elecsys Anti-SARS-CoV-2 chemiluminescence immunoassay. Clinical Chemistry and Laboratory Medicine 2020;58:e251-3. - PubMed
Liu 2020d {published data only}
-
- Liu Y, Liu Y, Diao B, Ren F, Wang Y, Ding J, et al. Diagnostic indexes of a rapid IgG/IgM combined antibody test for SARS-CoV-2. medRxiv [Preprint] 2020. [DOI: ]
Liu 2020e {published data only}
Liu 2020f {published data only}
-
- Liu R, Liu X, Han H, Shereen MA, Niu Z, Li D, et al. The comparative superiority of IgM-IgG antibody test to real-time reverse transcriptase PCR detection for SARS-CoV-2 infection diagnosis. medRxiv [Preprint] 2020. [DOI: ]
Lopez de la Iglesias 2020 {published data only}
Ma 2020a {published data only}
McAndrews 2020 {published data only}
-
- McAndrews KM, Dowlatshahi DP, Hensel J, Ostrosky-Zeichner LL, Papanna R, LeBleu VS, et al. Identification of IgG antibody response to SARS-CoV-2 spike protein and its receptor binding domain does not predict rapid recovery from COVID-19. medRxiv [Preprint] 2020. [DOI: ]
Morley 2020 {published data only}
Munitz 2020 {published data only}
-
- Munitz A, Edry-Botzer L, Itan M, Tur-Kaspa R, Dicker D, Markovitch D, et al. SARS-CoV-2 serological testing using electrochemiluminescence reveals arapid onset of seroconversion in severe COVID-19 patients. medRxiv [Preprint] 2020. [DOI: ]
Mutnal 2020 {published data only}
-
- Mutnal MB, Mohammad AA, Arroliga AC, Hua Y, Wang L, Koss W, et al. Role of Anti-SARS-CoV-2 antibodies in different cohorts: Can they provide clues for appropriate patient triaging? bioRxiv [Preprint] 2020. [DOI: ]
Nath 2020 {published data only}
-
- Nath H, Mallick A, Roy S, Sukla S, Basu K, De A, et al. Dengue antibodies can cross-react with SARS-CoV-2 and vice versa-Antibody detection kits can give false-positive results for both viruses in regions where both COVID-19 and Dengue co-exist. medRxiv [Preprint] 2020. [DOI: ]
Nguyen 2020a {published data only}
-
- Nguyen NN, Mutnal MB, Gomez RR, Pham HN, Nguyen LT, Koss W, et al. Correlation of ELISA based with random access serologic immunoassays for identifying adaptive immune response to SARS-CoV-2. medRxiv [Preprint] 2020;10. [DOI: ]
Nie 2020 {published data only}
Norman 2020 {published data only}
Nuccetelli 2020 {published data only}
Okba 2020a {published data only}
-
- Okba N, Muller MA, Li W, Wang C, GeurtsvanKessel CH, Corman VM, et al. SARS-CoV-2 specific antibody responses in COVID-19 patients. medRxiv [Preprint] 2020. [DOI: 10.1101/2020.03.18.20038059] - DOI
-
- Woelfel R, Corman VM, Guggemos W, Seilmaier M, Zange S, Muller MA, et al. Virological assessment of hospitalized patients with COVID-2019. Nature 2020;581:465-9. - PubMed
Olivares 2020 {published data only}
-
- Olivares F, Muñoz D, Fica A, Delama I, Alvarez I, Navarrete M, et al. Covid-19 in Chile. The experience of a Regional reference Center. Preliminary report. medRxiv [Preprint] 2020. [DOI: ] - PubMed
Ossareh 2020 {published data only}
-
- Ossareh S, Bagheri M, Abbasi M, Abolfathi S, Bohlooli A. Role of screening for COVID-19 in hemodialysis wards, results of a single center study. Iranian Journal of Kidney Diseases 2020;14(5):389-98. - PubMed
Ozturk 2020 {published data only}
-
- Ozturk T, Howell C, Benameur K, Ramonell RP, Cashman K, Pirmohammed S, et al. Cross-sectional IgM and IgG profiles in SARS-CoV-2 infection. medRxiv [Preprint] 2020. [DOI: ]
Paradiso 2020a {published data only}
-
- Paradiso AV, De Summa S, Loconsole D, Procacci V, Sallustio A, Centrone F, et al. Clinical meanings of rapid serological assay in patients tested for SARS-Co2 RT-PCR. medRxiv [Preprint] 2020. [DOI: ]
Paradiso 2020b {published data only}
-
- Paradiso AV, De Summa S, Silvestris N, Tommasi S, Tufaro A, De Palma G, et al. Rapid serological tests have a role in asymptomatic health workers COVID-19 screening. medRxiv [Preprint] 2020. [DOI: 10.1101/2020.04.15.20057786] - DOI
Patel 2020 {published data only}
Pellanda 2020 {published data only}
-
- Pellanda LC, da Ros Wendland EM, McBride AJA, Tovo-Rodrigues L, Ferreira MRA, Dellagostin OA, et al. Sensitivity and specificity of a rapid test for assessment of exposure to SARS-CoV-2 in a community-based setting in Brazil. medRxiv [Preprint] 2020. [DOI: ]
Perkmann 2020 {published data only}
Phan 2020 {published data only}
Plebani 2020 {published data only}
Prince 2020 {published data only}
Qian 2020 {published data only}
-
- Qian C, Zhou M, Cheng F, Lin X, Gong Y, Xie X, et al. Development and multicenter performance evaluation of the first fully automated SARS-CoV-2 IgM and IgG immunoassays. medRxiv [Preprint] 2020. [DOI: ] - PubMed
Qu 2020 {published data only}
Rabets 2020a {published data only}
Randad 2020 {published data only}
Robledo Gomez 2020 {published data only}
Rosado 2020 {published data only}
Rosendal 2020 {published data only}
-
- Rosendal E, Wigren J, Groening R, Yongdae K, Nilsson E, Sharma A, et al. Detection of asymptomatic SARS-CoV-2 exposed individuals by a sensitive S-based ELISA. medRxiv [Preprint] 2020. [DOI: ]
Rushworth 2020 {published data only}
-
- Rushworth SA, Johnson BB, Ashurst K, Davidson R, Paddy P, Mistry JJ, et al. Performance and health economic evaluation of the Mount Sinai COVID-19 serological assay identifies modification of thresholding as necessary to maximise specificity of the assay. medRxiv [Preprint] 2020. [DOI: ]
Santos 2020 {published data only}
Serrano 2020 {published data only}
Shaw 2020 {published data only}
-
- Shaw AM, Hyde C, Merrick B, James-Pemberton P, Squires BK, Olkhov RV, et al. Real-world evaluation of a novel technology for quantitative simultaneous antibody detection against multiple SARS-CoV-2 antigens in a cohort of patients presenting with COVID-19 syndrome. Analyst 2020;145(16):5638-46. - PMC - PubMed
Solodky 2020 {published data only}
Song 2020 {published data only}
Spicuzza 2020 {published data only}
Staines 2020 {published data only}
-
- Staines HM, Kirwan DE, Clark DJ, Adams ER, Augustin Y, Byrne RL, et al. Dynamics of IgG seroconversion and pathophysiology of COVID-19 infections. medRxiv [Preprint] 2020. [DOI: ]
Steiner 2020 {published data only}
Strömer 2020 {published data only}
-
- Strömer A, Grobe O, Rose R, Fickenscher H, Lorentz T, Krumbholz A. Diagnostic accuracy of six commercial SARS-CoV-2 IgG/total antibody assays and identification of SARS-CoV-2 neutralizing antibodies in convalescent sera. medRxiv [Preprint] 2020. [DOI: ]
Sun 2020a {published data only}
Tan 2020 {published data only}
Tan 2020a {published data only}
-
- Tan W, Lu Y, Zhang J, Wang J, Dan Y, Tan Z, et al. Viral kinetics and antibody responses in patients with COVID-19. medRxiv [Preprint] 2020. [DOI: ]
Teng 2020 {published data only}
Thevis 2020 {published data only}
To 2020a {published data only}
Tre‐Hardy 2020 {published data only}
Valenti 2020 {published data only}
Van Praet 2021 {published data only}
Varadhachary 2020 {published data only}
-
- Varadhachary A, Chatterjee D, Garza J, Garr RP, Foley C, Letkeman AF, et al. Salivary anti-SARS-CoV-2 IgA as an accessible biomarker of mucosal immunity against COVID-19. medRxiv [Preprint] 2020. [DOI: ]
Vasarhelyi 2020 {published data only}
-
- Vasarhelyi B, Kristof K, Ostorhazi E, Szabo D, Prohaszka Z, Merkely B. The diagnostic value of rapid anti IgM and IgG detecting tests in the identification of patients with SARS CoV-2 virus infection. Orvosi Hetilap 2020;161(20):807-12. - PubMed
Vidal‐Anzardo 2020 {published data only}
-
- Vidal-Anzardo M, Solis G, Solari L, Minaya G, Ayala-Quintanilla B, Astete-Cornejo J, et al. Evaluation of a rapid serological test for detection of IgM and igG antibodies against SARS-CoV-2 under field conditions. Revista Peruana de Medicina Experimental y Salud Publica 2020;37(2):203-9. - PubMed
Villarreal 2020 {published data only}
Wajnberg 2020 {published data only}
-
- Wajnberg A, Mansour M, Leven E, Bouvier NM, Patel G, Firpo A, et al. Humoral immune response and prolonged PCR positivity in a cohort of 1343 SARS-CoV 2 patients in the New York City region. medRxiv [Preprint] 2020. [DOI: ]
Wan 2020 {published data only}
Wang 2020b {published data only}
-
- Wang Q, Du Q, Guo B, Guo Y, Fang L, X Guo. Performance of urea-mediated dissociation in reducing false-positive of 2019-nCoV IgM test. Chinese Journal of Laboratory Medicine 2020;43:889-93. [DOI: 10.3760/cma.j.cn114452-20200219-00091] - DOI
Wang 2020c {published data only}
-
- Wang Z, Li H, Li J, Yang C, Guo X, Hu Z, et al. Elevated serum IgM levels indicate poor outcome in patients with coronavirus disease 2019 pneumonia: a retrospective case-control study. medRxiv [Preprint] 2020. [DOI: ]
Wang 2020d {published data only}
-
- Wang X, Guo X, Xin Q, Chu Y, Li J, Pan Y, et al. Neutralizing antibodies responses to SARS-CoV-2 in COVID-19 inpatients and convalescent patients. medRxiv [Preprint] 2020. [DOI: ]
Wang 2020e {published data only}
-
- Wang B, Wang L, Kong X, Geng J, Xiao D, Ma C. Long-term coexistence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) with antibody response in non-severe coronavirus disease 2019 (COVID-19) patients. medRxiv [Preprint] 2020. [DOI: ]
Wechselberger 2020 {published data only}
Weiss 2020 {published data only}
Wen 2020 {published data only}
-
- Wen T, Huang C, Shi FJ, Zeng XY, Lu T, Ding SN, et al. Development of a lateral flow immunoassay strip for rapid detection of IgG antibody against SARS-CoV-2 virus. Analyst 2020;145(15):5345-52. - PubMed
Wheeler 2020 {published data only}
Woelfel 2020 {published data only}
-
- Woelfel R, Corman VM, Guggemos W, Seilmaier M, Zange S, Mueller MA, et al. Clinical presentation and virological assessment of hospitalized cases of coronavirus disease 2019 in a travel-associated transmission cluster. medRxiv [Preprint] 2020. [DOI: ]
Wu 2020a {published data only}
Xiang 2020b {published data only}
-
- Xiang J, Yan M, Li H, Liu T, Lin C, Huang S, et al. Evaluation of enzyme-linked immunoassay and colloidal gold- immunochromatographic assay kit for detection of novel coronavirus (SARS-Cov-2) causing an outbreak of pneumonia (COVID-19). medRxiv [Preprint] 2020. [DOI: ]
Xie 2020a {published data only}
Xu 2020a {published data only}
-
- Xu Y. Dynamic profile of severe or critical COVID-19 cases. medRxiv [Preprint] 2020. [DOI: ]
Xu 2020b {published data only}
Xu 2020c {published data only}
Xue 2020 {published data only}
Yamaoka 2021 {published data only}
Yan 2021 {published data only}
Yildirim 2020 {published data only}
Yokoyama 2020 {published data only}
Yu 2020 {published data only}
Yue 2020 {published data only}
-
- Yue H, Nowak RP, Overwijn D, Payne NC, Fischinger S, Atyeo C, et al. Rapid 'mix and read' assay for scalable detection of SARS-CoV-2 antibodies in patient plasma. medRxiv [Preprint] 2020. [DOI: ]
Zeng 2020a {published data only}
Zeng 2020b {published data only}
Zhang 2020c {published data only}
Zhang 2020d {published data only}
-
- Zhang P, Gao Q, Wang T, Ke Y, Mo F, Jia R, et al. Evaluation of recombinant nucleocapsid and spike proteins for serological diagnosis of novel coronavirus disease 2019 (COVID-19). medRxiv [Preprint] 2020. [DOI: ]
Zhang 2020e {published data only}
Zhang 2020f {published data only}
Zhao 2020b {published data only}
-
- Zhao R, Li M, Song H, Chen J, Ren W, Feng Y, et al. Serological diagnostic kit of SARS-CoV-2 antibodies using CHO-expressed full-length SARS-CoV-2 S1 proteins. medRxiv [Preprint] 2020. [DOI: ]
Zhong 2020 {published data only}
Zhou 2020 {published data only}
-
- Zhou Q, Zhu D, Yan H, Quan J, Kuang Z, Zhang W, et al. A preliminary study on analytical performance of serological assay for SARS-CoV-2 IgM/IgG and application in clinical practice. medRxiv [Preprint] 2020. [DOI: ]
Additional references
Agarwal 2020
-
- Agarwal A, Rochwerg B, Lamontagne F, Siemieniuk RA, Agoritsas T, Askie L, et al. A living WHO guideline on drugs for covid-19 (last update Mar 3 2022). BMJ 2020;370:m3379. - PubMed
Arevalo‐Rodriguez 2020a
Bonanni 2021
-
- Bonanni P, Cantón R, Gill D, Halfon P, Liebert UG, Crespo KAN, et al. The role of serology testing to strengthen vaccination initiatives and policies for COVID-19 in Europe. COVID 2021;1(1):20-38.
Bossuyt 2015
CDC 2020a
-
- Centers for Disease Control and Prevention (CDC). Multisystem Inflammatory Syndrome in Adults (MIS-A) Case definition information for healthcare providers (last reviewed November 13, 2020). Available from: cdc.gov/mis/mis-a (accessed 31 March 2021).
CDC 2021a
-
- Centers for Disease Control and Prevention (CDC). Information for Healthcare Providers about Multisystem Inflammatory Syndrome in Children (MIS-C) (last reviewed May 20, 2021). Available from: cdc.gov/mis/mis-c/hcp/index.html (accessed 31 March 2021).
CDC 2021b
-
- Centers for Disease Control and Prevention (CDC). Interim Guidelines for COVID-19 Antibody Testing (updated September 21, 2021). Available from: www.cdc.gov/coronavirus/2019-ncov/lab/resources/antibody-tests-guideline... (accessed 9 December 2021).
CDC China 2020
-
- National Health Commission of the People's Republic of China. Release of 7th edition of case definitions. www.nhc.gov.cn/yzygj/s7653p/202003/46c9294a7dfe4cef80dc7f5912eb1989.shtml (accessed prior to 23 June 2020).
Chua 2021
-
- Chua CRR, los Santos EDE, Escasa KVH, Estolas RLG, Feliciano J, Ortega SAE, et al. Diagnostic accuracy of COVID-19 antibody tests authorized by FDA Philippines: a systematic review and meta-analysis. SciMedicine Journal 2021;3(4):283-301.
Corman 2020
COVID‐19 Open Access Project 2021
-
- COVID-19 Open Access Project. Living Evidence on COVID-19. Available at ispmbern.github.io/covid-19/living-review/.
Covidence [Computer program]
-
- Covidence. Version accessed 27 April 2020. Melbourne, Australia: Veritas Health Innovation. Available at covidence.org.
De Carvalho 2021
-
- Carvalho CC, Cardozo MM, Gonelli R, Regueira SL, Souza AB, Ramos IB, et al. Diagnostic accuracy of serological tests for COVID-19: a systematic review and meta-analysis of cohort studies. La Rivista Italiana della Medicina di Laboratorio 2021;17(4):227-36. [DOI: 10.23736/S1825-859X.21.00122-5] - DOI
Dinnes 2021
Doust 2021
-
- Doust JA, Bell KJL, Leeflang MMG, Dinnes J, Lord SJ, Mallett S, et al. Guidance for the design and reporting of studies evaluating the clinical performance of tests for present or past SARS-CoV-2 infection. BMJ 2021;372:n568. - PubMed
ECDC 2021
-
- European Centre for Disease Prevention and Control. The use of antibody tests for SARS-COV-2 in the context of Digital Green Certificates; 10 May 2021. Available at https://www.ecdc.europa.eu/sites/default/files/documents/Use-of-antibody....
FDA 2021a
-
- FDA. SARS-CoV-2 viral mutations: impact on COVID-19 tests. https://www.fda.gov/medical-devices/coronavirus-covid-19-and-medical-dev... (accessed 31 March 2022).
FDA 2022
-
- FDA. Coronavirus (COVID-19) Update: FDA authorizes new monoclonal antibody for treatment of COVID-19 that retains activity against omicron variant (Feb 11 2022). Available at www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-....
Gracienta 2022
Greaney 2022
Healy 2021
HIQA 2021
-
- Health Information and Quality Authority. Duration of protective immunity (protection from reinfection) following SARS-CoV-2 infection; updated on 18 Nov 2021. https://www.hiqa.ie/reports-and-publications/health-technology-assessmen....
Islam 2021
Junker 2022
-
- Junker D, Becker M, Wagner TR, Kaiser PD, Maier S, Grimm TM, et al. Antibody binding and ACE2 binding inhibition is significantly reduced for the Omicron variant compared to all other variants of concern. medRxiv [Preprint] 2021. [DOI: ]
Kucirka 2020
Kumar 2021
-
- Kumar M, Swarnim S, Pallavi P. Clinical characteristics of multisystem inflammatory syndrome in children and young adults with COVID-19: a rapid systematic review. Journal of Pediatrics Review 2022;10:367-88.
Leeflang 2021
-
- Leeflang MMG, Bell K, Deeks JJ, Dinnes J, Doust J, Korevaar DA, et al. Electronic and animal noses for detecting SARS-CoV-2 infection. Cochrane Database of Systematic Reviews 2021, Issue 6. Art. No: CD015013. [DOI: 10.1002/14651858.CD015013] - DOI
Macaskill 2010
-
- Macaskill P, Gatsonis C, Deeks JJ, Harbord RM, Takwoingi Y. Chapter 10: analysing and presenting results. In: Deeks JJ, Bossuyt PM, Gatsonis C editor(s). Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy Version 1.0. The Cochrane Collaboration, 2010. Available from methods.cochrane.org/sdt/handbook-dta-reviews.
Macedo 2022
Makoah 2022
Mayers 2020
-
- Mayers C, Baker K, Government Office for Science. Impact of false-positives and false-negatives in the UK’s COVID-19 RT-PCR testing programme; 3 June 2020. Available at assets.publishing.service.gov.uk/government/uploads/system/uploads/attac....
McInnes 2018
McInnes 2020
MHRA 2021a
-
- Medicines and Healthcare Products Regulatory Authority. Target product profile: Antibody tests to help determine if people have immunity to SARS-CoV-2 (v2; updated 14 February 2022). Available at www.gov.uk/government/publications/how-tests-and-testing-kits-for-corona....
MHRA 2021b
-
- Medicines and Healthcare Products Regulatory Authority. Target product profile: Enzyme immunoassay (EIA) antibody tests to help determine if people have antibodies to SARS-CoV-2 (v2; updated 14 February 2022). Available at www.gov.uk/government/publications/how-tests-and-testing-kits-for-corona....
Moher 2009
ONS 2020
-
- Office for National Statistics. Coronavirus (COVID-19) Infection Survey, UK: 21 August 2020. Available at ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsan....
Patel 2021
Post 2021
Riley 2020
-
- Riley S, Ainslie KE, Eales O, Walters CE, Wang H, Atchison C, et al, REACT Study Investigators. Transient dynamics of SARS-CoV-2 as England exited national lockdown. medRxiv [Preprint] 2020;.. [DOI: 10.1101/2020.08.05.20169078] [DOI: ] - DOI
RSS 2021
-
- Royal Statistical Society. Royal Statistical Society Diagnostic Tests Working Group Report; June 2021. Available at https://rss.org.uk/RSS/media/File-library/Policy/2021/RSS-Diagnostic-tes....
Stata [Computer program]
-
- Stata. Version 17. College Station, TX, USA: StataCorp, 2021. Available at www.stata.com.
Stegeman 2020
Struyf 2021
-
- Struyf T, Deeks JJ, Dinnes J, Takwoingi Y, Davenport C, Leeflang MM, et al. Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19. Cochrane Database of Systematic Reviews 2021, Issue 2. Art. No: CD013665. [DOI: 10.1002/14651858.CD013665.pub2] - DOI - PMC - PubMed
Takwoingi 2017
Veroniki 2022
-
- Veroniki AA, Tsokani S, Agarwal R, Pagkalidou E, Rücker G, Mavridis D, et al. Diagnostic test accuracy network meta-analysis methods: A scoping review and empirical assessment. Journal of Clinical Epidemiology 2022;146:86-96. - PubMed
Ward 2022
West 2021
Whiting 2011
-
- Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Annals of Internal Medicine 2011;155(8):529-36. - PubMed
WHO 2020
-
- World Health Organization (WHO). Global surveillance for COVID-19 caused by human infection with COVID-19 virus Interim guidance. www.who.int/publications/i/item/global-surveillance-for-human-infection-... (accessed 23 June 2020). [https://www.who.int/publications/i/item/global-surveillance-for-human-in...]
Yadav 2022
Yang 2020a
Yang 2021
-
- Yang B, Mallett S, Takwoingi Y, Davenport CF, Hyde CJ, Whiting PF, et al. QUADAS-C: a tool for assessing risk of bias in comparative diagnostic accuracy studies. Annals of Internal Medicine 2021;174(11):1592-9. - PubMed
References to other published versions of this review
Deeks 2020a
Deeks 2020b
-
- Deeks JJ, Dinnes J, Takwoingi Y, Davenport C, Leeflang MM, Spijker R, et al. Diagnosis of SARS-CoV-2 infection and COVID-19: accuracy of signs and symptoms; molecular, antigen, and antibody tests; and routine laboratory markers. Cochrane Database of Systematic Reviews 2020, Issue 4. Art. No: CD013596. [DOI: 10.1002/14651858.CD013596] - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

