Effect of Biogenic Silver Nanoparticles on the Quorum-Sensing System of Pseudomonas aeruginosa PAO1 and PA14
- PMID: 36144357
- PMCID: PMC9504124
- DOI: 10.3390/microorganisms10091755
Effect of Biogenic Silver Nanoparticles on the Quorum-Sensing System of Pseudomonas aeruginosa PAO1 and PA14
Abstract
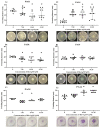
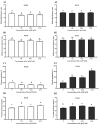
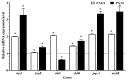
The increase in multidrug-resistant microorganisms represents a global threat requiring the development novel strategies to fight bacterial infection. This study aimed to assess the effect of silver nanoparticles (bio-AgNPs) on bacterial growth, biofilm formation, production of virulence factors, and expression of genes related to the quorum-sensing (QS) system of P. aeruginosa PAO1 and PA14. Biofilm formation and virulence assays were performed with bio-AgNPs. RT-qPCR was carried out to determine the effect of bio-AgNPs on the QS regulatory genes lasI, lasR, rhlI, rhlR, pqsA, and mvfR. Bio-AgNPs had an MIC value of 62.50 μM, for both strains. Phenotypic and genotypic assays were carried out using sub-MIC values. Experimental results showed that treatment with sub-MICs of bio-AgNPs reduced (p < 0.05) the motility and rhamnolipids and elastase production in P. aeruginosa PAO1. In PA14, bio-AgNPs stimulated swarming and twitching motilities as well as biofilm formation and elastase and pyocyanin production. Bio-AgNP treatment increased (p < 0.05) the expression of QS genes in PAO1 and PA14. Despite the different phenotypic behaviors in both strains, both showed an increase in the expression of QS genes. Demonstrating that the bio-AgNPs acted in the induction of regulation. The possible mechanism underlying the action of bio-AgNPs involves the induction of the rhl and/or pqs system of PAO1 and of the las and/or pqs system of PA14. These results suggest that exposure to low concentrations of bio-AgNPs may promote the expression of QS regulatory genes in P. aeruginosa, consequently inducing the production of virulence factors such as elastase, pyocyanin, and biofilms.
Keywords: AgNPs; antivirulence; gene regulation; quorum quenching.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Subinhibitory Concentrations of Biogenic Silver Nanoparticles Affect Motility and Biofilm Formation in Pseudomonas aeruginosa.Front Cell Infect Microbiol. 2021 Apr 1;11:656984. doi: 10.3389/fcimb.2021.656984. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 33869087 Free PMC article.
-
The Small RNA AmiL Regulates Quorum Sensing-Mediated Virulence in Pseudomonas aeruginosa PAO1.Microbiol Spectr. 2022 Apr 27;10(2):e0221121. doi: 10.1128/spectrum.02211-21. Epub 2022 Mar 9. Microbiol Spectr. 2022. PMID: 35262393 Free PMC article.
-
Diallyl disulfide from garlic oil inhibits Pseudomonas aeruginosa virulence factors by inactivating key quorum sensing genes.Appl Microbiol Biotechnol. 2018 Sep;102(17):7555-7564. doi: 10.1007/s00253-018-9175-2. Epub 2018 Jun 27. Appl Microbiol Biotechnol. 2018. PMID: 29951860
-
Sesamin and sesamolin rescues Caenorhabditis elegans from Pseudomonas aeruginosa infection through the attenuation of quorum sensing regulated virulence factors.Microb Pathog. 2021 Jun;155:104912. doi: 10.1016/j.micpath.2021.104912. Epub 2021 Apr 28. Microb Pathog. 2021. PMID: 33932548 Review.
-
Bile effects on the Pseudomonas aeruginosa pathogenesis in cystic fibrosis patients with gastroesophageal reflux.Heliyon. 2023 Nov 10;9(11):e22111. doi: 10.1016/j.heliyon.2023.e22111. eCollection 2023 Nov. Heliyon. 2023. PMID: 38034726 Free PMC article. Review.
Cited by
-
Green Biofabrication of Silver Nanoparticles of Potential Synergistic Activity with Antibacterial and Antifungal Agents against Some Nosocomial Pathogens.Microorganisms. 2023 Apr 4;11(4):945. doi: 10.3390/microorganisms11040945. Microorganisms. 2023. PMID: 37110368 Free PMC article.
-
A review on mycogenic metallic nanoparticles and their potential role as antioxidant, antibiofilm and quorum quenching agents.Heliyon. 2024 Apr 16;10(8):e29500. doi: 10.1016/j.heliyon.2024.e29500. eCollection 2024 Apr 30. Heliyon. 2024. PMID: 38660254 Free PMC article. Review.
-
How Nanoparticles Help in Combating Chronic Wound Biofilms Infection?Int J Nanomedicine. 2024 Nov 15;19:11883-11921. doi: 10.2147/IJN.S484473. eCollection 2024. Int J Nanomedicine. 2024. PMID: 39563901 Free PMC article. Review.
-
The two faces of pyocyanin - why and how to steer its production?World J Microbiol Biotechnol. 2023 Feb 18;39(4):103. doi: 10.1007/s11274-023-03548-w. World J Microbiol Biotechnol. 2023. PMID: 36864230 Free PMC article. Review.
-
Synergistic Antibacterial Proficiency of Green Bioformulated Zinc Oxide Nanoparticles with Potential Fosfomycin Synergism against Nosocomial Bacterial Pathogens.Microorganisms. 2023 Mar 2;11(3):645. doi: 10.3390/microorganisms11030645. Microorganisms. 2023. PMID: 36985218 Free PMC article.
References
-
- Tacconelli E., Carrara E., Savoldi A., Harbarth S., Mendelson M., Monnet D.L., Pulcini C., Kahlmeter G., Kluytmans J., Carmeli Y., et al. Discovery, research, and development of new antibiotics: The WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect. Dis. 2018;18:318–327. doi: 10.1016/S1473-3099(17)30753-3. - DOI - PubMed
-
- Fleitas Martínez O., Cardoso M.H., Ribeiro S.M., Franco O.L. Recent Advances in Anti-virulence Therapeutic Strategies with a Focus on Dismantling Bacterial Membrane Microdomains, Toxin Neutralization, Quorum-Sensing Interference and Biofilm Inhibition. Front. Cell. Infect. Microbiol. 2019;2:74. doi: 10.3389/fcimb.2019.00074. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

