A randomized, double-blind trial of triheptanoin for drug-resistant epilepsy in glucose transporter 1 deficiency syndrome
- PMID: 35441706
- PMCID: PMC9546029
- DOI: 10.1111/epi.17263
A randomized, double-blind trial of triheptanoin for drug-resistant epilepsy in glucose transporter 1 deficiency syndrome
Abstract
Objective: This study was undertaken to evaluate efficacy and long-term safety of triheptanoin in patients >1 year old, not on a ketogenic diet, with drug-resistant seizures associated with glucose transporter 1 deficiency syndrome (Glut1DS).
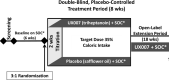
Methods: UX007G-CL201 was a randomized, double-blind, placebo-controlled trial. Following a 6-week baseline period, eligible patients were randomized 3:1 to triheptanoin or placebo. Dosing was titrated to 35% of total daily calories over 2 weeks. After an 8-week placebo-controlled period, all patients received open-label triheptanoin through Week 52.
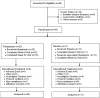
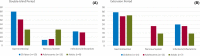
Results: The study included 36 patients (15 children, 13 adolescents, eight adults). A median 12.6% reduction in overall seizure frequency was observed in the triheptanoin arm relative to baseline, and a 13.5% difference was observed relative to placebo (p = .58). In patients with absence seizures only (n = 9), a median 62.2% reduction in seizure frequency was observed in the triheptanoin arm relative to baseline. Only one patient with absence seizures only was present in the control group, preventing comparison. No statistically significant differences in seizure frequency were observed. Common treatment-emergent adverse events included diarrhea, vomiting, abdominal pain, and nausea, mostly mild or moderate in severity. No serious adverse events were considered to be treatment related. One patient discontinued due to status epilepticus.
Significance: Triheptanoin did not significantly reduce seizure frequency in patients with Glut1DS not on the ketogenic diet. Treatment was associated with mild to moderate gastrointestinal treatment-related events; most resolved following dose reduction or interruption and/or medication for treatment. Triheptanoin was not associated with any long-term safety concerns when administered at dose levels up to 35% of total daily caloric intake for up to 1 year.
Trial registration: ClinicalTrials.gov NCT01993186.
Keywords: diet treatment; drug resistance; epilepsy; glucose transporter 1 deficiency syndrome; triheptanoin.
© 2022 The Authors. Epilepsia published by Wiley Periodicals LLC on behalf of International League Against Epilepsy.
Conflict of interest statement
P.S. has received fees from Ultragenyx Pharmaceutical Inc, Zogenix, BioMarin, PTC Therapeutics, GW Pharma, and Neuraxpharm, and research grants from GW Pharma, PTC Therapeutics, Enecta, and Kolfarma. He has been an investigator for clinical trials for Ultragenyx Pharmaceutical Inc and Zogenix. He has served on a scientific advisory board for the Italian Medicines Agency; has received honoraria from GW Pharma, Kolfarma, and Eisai; and has received research support from the Italian Ministry of Health and San Paolo Foundation. S.A. has served as a consultant or received honoraria for lectures from Biocodex, BioMarin, Eisai, GW Pharma, Neuraxpharm, Nutricia, UCB Pharma, Ultragenyx Pharmaceutical Inc, and Zogenix. He has been an investigator for clinical trials for Eisai, UCB Pharma, Ultragenyx Pharmaceutical Inc, and Zogenix. I.E.S. has served on scientific advisory boards for UCB, Eisai, GlaxoSmithKline, BioMarin, Nutricia, Rogcon, Chiesi, Encoded Therapeutics, Knopp Biosciences, and Xenon Pharmaceuticals; has received speaker honoraria from GlaxoSmithKline, UCB, BioMarin, Biocodex, Chiesi, LivaNova, and Eisai; has received funding for travel from UCB, Biocodex, GlaxoSmithKline, BioMarin, and Eisai; has served as an investigator for Zogenix, Zynerba, Ultragenyx Pharmaceutical Inc, GW Pharma, UCB, Eisai, Xenon Pharmaceuticals, Anavex Life Sciences, Ovid Therapeutics, Epygenix Therapeutics, Encoded Therapeutics, and Marinus; and has consulted for Zynerba Pharmaceuticals, Atheneum Partners, Ovid Therapeutics, Care Beyond Diagnosis, Epilepsy Consortium, and UCB. She may accrue future revenue on pending patent WO61/010176 (filed 2008; Therapeutic Compound); has a patent for
Figures
Similar articles
-
Lamotrigine adjunctive therapy among children and adolescents with primary generalized tonic-clonic seizures.Pediatrics. 2006 Aug;118(2):e371-8. doi: 10.1542/peds.2006-0148. Epub 2006 Jul 17. Pediatrics. 2006. PMID: 16847080 Clinical Trial.
-
Efficacy and safety of perampanel in patients with drug-resistant partial seizures after conversion from double-blind placebo to open-label perampanel.Epilepsy Res. 2015 Aug;114:131-40. doi: 10.1016/j.eplepsyres.2015.04.011. Epub 2015 May 1. Epilepsy Res. 2015. PMID: 26088896 Clinical Trial.
-
Triheptanoin for glucose transporter type I deficiency (G1D): modulation of human ictogenesis, cerebral metabolic rate, and cognitive indices by a food supplement.JAMA Neurol. 2014 Oct;71(10):1255-65. doi: 10.1001/jamaneurol.2014.1584. JAMA Neurol. 2014. PMID: 25110966 Free PMC article.
-
Rufinamide add-on therapy for refractory epilepsy.Cochrane Database Syst Rev. 2018 Apr 25;4(4):CD011772. doi: 10.1002/14651858.CD011772.pub2. Cochrane Database Syst Rev. 2018. Update in: Cochrane Database Syst Rev. 2020 Nov 8;11:CD011772. doi: 10.1002/14651858.CD011772.pub3. PMID: 29691835 Free PMC article. Updated. Review.
-
GLUT1 deficiency syndrome in clinical practice.Epilepsy Res. 2012 Jul;100(3):272-7. doi: 10.1016/j.eplepsyres.2011.02.007. Epub 2011 Mar 5. Epilepsy Res. 2012. PMID: 21382692 Review.
Cited by
-
Combination of triheptanoin with the ketogenic diet in Glucose transporter type 1 deficiency (G1D).Sci Rep. 2023 Jun 2;13(1):8951. doi: 10.1038/s41598-023-36001-x. Sci Rep. 2023. PMID: 37268656 Free PMC article.
-
Clinical, biochemical and molecular characterization of 12 patients with pyruvate carboxylase deficiency treated with triheptanoin.Mol Genet Metab. 2023 Jun;139(2):107605. doi: 10.1016/j.ymgme.2023.107605. Epub 2023 May 9. Mol Genet Metab. 2023. PMID: 37207470 Free PMC article.
-
GLUT-1DS resistant to ketogenic diet: from clinical feature to in silico analysis. An exemplificative case report with a literature review.Neurogenetics. 2024 Apr;25(2):69-78. doi: 10.1007/s10048-023-00742-8. Epub 2024 Jan 8. Neurogenetics. 2024. PMID: 38190079 Review.
-
"Brain propane'': Propionate Fuels the Brain in Mice With Brain Energy Deficits.Epilepsy Curr. 2024 Nov 13:15357597241293843. doi: 10.1177/15357597241293843. Online ahead of print. Epilepsy Curr. 2024. PMID: 39545015 Free PMC article. No abstract available.
-
GLUT1-DS Italian registry: past, present, and future: a useful tool for rare disorders.Orphanet J Rare Dis. 2023 Mar 21;18(1):63. doi: 10.1186/s13023-023-02628-2. Orphanet J Rare Dis. 2023. PMID: 36944981 Free PMC article.
References
-
- Pearson TS, Akman C, Hinton VJ, Engelstad K, De Vivo DC. Phenotypic spectrum of glucose transporter type 1 deficiency syndrome (Glut1 DS). Curr Neurol Neurosci Rep. 2013;13(4):342. - PubMed
-
- De Vivo DC, Trifiletti RR, Jacobson RI, Ronen GM, Behmand RA, Harik SI. Defective glucose transport across the blood‐brain barrier as a cause of persistent hypoglycorrhachia, seizures, and developmental delay. N Engl J Med. 1991;325(10):703–9. - PubMed
-
- Koch H, Weber YG. The glucose transporter type 1 (Glut1) syndromes. Epilepsy Behav. 2019;91:90–3. - PubMed
-
- Coman DJ, Sinclair KG, Burke CJ, Appleton DB, Pelekanos JT, O'Neil CM, et al. Seizures, ataxia, developmental delay and the general paediatrician: glucose transporter 1 deficiency syndrome. J Paediatr Child Health. 2006;42(5):263–7. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Associated data
LinkOut - more resources
Full Text Sources

