Peroxisomal Stress Response and Inter-Organelle Communication in Cellular Homeostasis and Aging
- PMID: 35204075
- PMCID: PMC8868334
- DOI: 10.3390/antiox11020192
Peroxisomal Stress Response and Inter-Organelle Communication in Cellular Homeostasis and Aging
Abstract
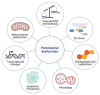
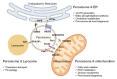
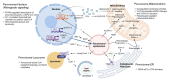
Peroxisomes are key regulators of cellular and metabolic homeostasis. These organelles play important roles in redox metabolism, the oxidation of very-long-chain fatty acids (VLCFAs), and the biosynthesis of ether phospholipids. Given the essential role of peroxisomes in cellular homeostasis, peroxisomal dysfunction has been linked to various pathological conditions, tissue functional decline, and aging. In the past few decades, a variety of cellular signaling and metabolic changes have been reported to be associated with defective peroxisomes, suggesting that many cellular processes and functions depend on peroxisomes. Peroxisomes communicate with other subcellular organelles, such as the nucleus, mitochondria, endoplasmic reticulum (ER), and lysosomes. These inter-organelle communications are highly linked to the key mechanisms by which cells surveil defective peroxisomes and mount adaptive responses to protect them from damages. In this review, we highlight the major cellular changes that accompany peroxisomal dysfunction and peroxisomal inter-organelle communication through membrane contact sites, metabolic signaling, and retrograde signaling. We also discuss the age-related decline of peroxisomal protein import and its role in animal aging and age-related diseases. Unlike other organelle stress response pathways, such as the unfolded protein response (UPR) in the ER and mitochondria, the cellular signaling pathways that mediate stress responses to malfunctioning peroxisomes have not been systematically studied and investigated. Here, we coin these signaling pathways as "peroxisomal stress response pathways". Understanding peroxisomal stress response pathways and how peroxisomes communicate with other organelles are important and emerging areas of peroxisome research.
Keywords: ER stress; acetyl-CoA; apoptosis; mitochondrial dysfunction; peroxisome; pexophagy; plasmalogen; reactive oxygen species.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Mitochondria are targets for peroxisome-derived oxidative stress in cultured mammalian cells.Free Radic Biol Med. 2013 Dec;65:882-894. doi: 10.1016/j.freeradbiomed.2013.08.173. Epub 2013 Aug 27. Free Radic Biol Med. 2013. PMID: 23988789
-
Peroxisomal regulation of energy homeostasis: Effect on obesity and related metabolic disorders.Mol Metab. 2022 Nov;65:101577. doi: 10.1016/j.molmet.2022.101577. Epub 2022 Aug 19. Mol Metab. 2022. PMID: 35988716 Free PMC article. Review.
-
Organelle interplay-peroxisome interactions in health and disease.J Inherit Metab Dis. 2020 Jan;43(1):71-89. doi: 10.1002/jimd.12083. Epub 2019 Apr 16. J Inherit Metab Dis. 2020. PMID: 30864148 Free PMC article. Review.
-
Redox Regulation of Homeostasis and Proteostasis in Peroxisomes.Physiol Rev. 2018 Jan 1;98(1):89-115. doi: 10.1152/physrev.00033.2016. Physiol Rev. 2018. PMID: 29167332 Free PMC article. Review.
-
Organelle interplay in peroxisomal disorders.Trends Mol Med. 2009 Jul;15(7):293-302. doi: 10.1016/j.molmed.2009.05.002. Epub 2009 Jun 26. Trends Mol Med. 2009. PMID: 19560974 Review.
Cited by
-
The Third Annual Symposium of the Midwest Aging Consortium.J Gerontol A Biol Sci Med Sci. 2024 Feb 1;79(2):glad239. doi: 10.1093/gerona/glad239. J Gerontol A Biol Sci Med Sci. 2024. PMID: 37804247 Free PMC article.
-
Homeostasis control in health and disease by the unfolded protein response.Nat Rev Mol Cell Biol. 2025 Mar;26(3):193-212. doi: 10.1038/s41580-024-00794-0. Epub 2024 Nov 5. Nat Rev Mol Cell Biol. 2025. PMID: 39501044 Review.
-
Mitochondrial Dysfunction in Glycogen Storage Disorders (GSDs).Biomolecules. 2024 Sep 1;14(9):1096. doi: 10.3390/biom14091096. Biomolecules. 2024. PMID: 39334863 Free PMC article. Review.
-
Inhibition of VHL by VH298 Accelerates Pexophagy by Activation of HIF-1α in HeLa Cells.Molecules. 2024 Jan 18;29(2):482. doi: 10.3390/molecules29020482. Molecules. 2024. PMID: 38257395 Free PMC article.
-
Lipid and Transcriptional Regulation in a Parkinson's Disease Mouse Model by Intranasal Vesicular and Hexosomal Plasmalogen-Based Nanomedicines.Adv Healthc Mater. 2024 Jun;13(14):e2304588. doi: 10.1002/adhm.202304588. Epub 2024 Feb 28. Adv Healthc Mater. 2024. PMID: 38386974 Free PMC article.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

