DICER1 tumor predisposition syndrome: an evolving story initiated with the pleuropulmonary blastoma
- PMID: 34599283
- PMCID: PMC8695383
- DOI: 10.1038/s41379-021-00905-8
DICER1 tumor predisposition syndrome: an evolving story initiated with the pleuropulmonary blastoma
Abstract
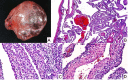
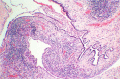
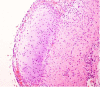
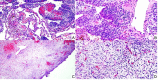
DICER1 syndrome (OMIM 606241, 601200) is a rare autosomal dominant familial tumor predisposition disorder with a heterozygous DICER1 germline mutation. The most common tumor seen clinically is the pleuropulmonary blastoma (PPB), a lung neoplasm of early childhood which is classified on its morphologic features into four types (IR, I, II and III) with tumor progression over time within the first 4-5 years of life from the prognostically favorable cystic type I to the unfavorable solid type III. Following the initial report of PPB, its association with other cystic neoplasms was demonstrated in family studies. The detection of the germline mutation in DICER1 provided the opportunity to identify and continue to recognize a number seemingly unrelated extrapulmonary neoplasms: Sertoli-Leydig cell tumor, gynandroblastoma, embryonal rhabdomyosarcomas of the cervix and other sites, multinodular goiter, differentiated and poorly differentiated thyroid carcinoma, cervical-thyroid teratoma, cystic nephroma-anaplastic sarcoma of kidney, nasal chondromesenchymal hamartoma, intestinal juvenile-like hamartomatous polyp, ciliary body medulloepithelioma, pituitary blastoma, pineoblastoma, primary central nervous system sarcoma, embryonal tumor with multilayered rosettes-like cerebellar tumor, PPB-like peritoneal sarcoma, DICER1-associated presacral malignant teratoid neoplasm and other non-neoplastic associations. Each of these neoplasms is characterized by a second somatic mutation in DICER1. In this review, we have summarized the salient clinicopathologic aspects of these tumors whose histopathologic features have several overlapping morphologic attributes particularly the primitive mesenchyme often with rhabdomyoblastic and chondroid differentiation and an uncommitted spindle cell pattern. Several of these tumors have an initial cystic stage from which there is progression to a high grade, complex patterned neoplasm. These pathologic findings in the appropriate clinical setting should serve to alert the pathologist to the possibility of a DICER1-associated neoplasm and initiate appropriate testing on the neoplasm and to alert the clinician about the concern for a DICER1 mutation.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures






Similar articles
-
Pleuropulmonary blastoma: a report on 350 central pathology-confirmed pleuropulmonary blastoma cases by the International Pleuropulmonary Blastoma Registry.Cancer. 2015 Jan 15;121(2):276-85. doi: 10.1002/cncr.29032. Epub 2014 Sep 10. Cancer. 2015. PMID: 25209242 Free PMC article.
-
An update on the central nervous system manifestations of DICER1 syndrome.Acta Neuropathol. 2020 Apr;139(4):689-701. doi: 10.1007/s00401-019-01997-y. Epub 2019 Apr 5. Acta Neuropathol. 2020. PMID: 30953130 Review.
-
Nasal chondromesenchymal hamartomas arise secondary to germline and somatic mutations of DICER1 in the pleuropulmonary blastoma tumor predisposition disorder.Hum Genet. 2014 Nov;133(11):1443-50. doi: 10.1007/s00439-014-1474-9. Epub 2014 Aug 14. Hum Genet. 2014. PMID: 25118636 Free PMC article.
-
Pleuropulmonary Blastoma: Evolution of an Entity as an Entry into a Familial Tumor Predisposition Syndrome.Pediatr Dev Pathol. 2015 Nov-Dec;18(6):504-11. doi: 10.2350/15-10-1732-OA.1. Epub 2015 Dec 23. Pediatr Dev Pathol. 2015. PMID: 26698637 Free PMC article.
-
DICER1 pleuropulmonary blastoma familial tumour predisposition syndrome: What the paediatric urologist needs to know.J Pediatr Urol. 2016 Feb;12(1):5-10. doi: 10.1016/j.jpurol.2015.08.012. Epub 2015 Sep 26. J Pediatr Urol. 2016. PMID: 26454454 Review.
Cited by
-
Expanding the spectrum of "mesenchymal" tumors of the central nervous system.Pathologica. 2022 Dec;114(6):455-464. doi: 10.32074/1591-951X-826. Pathologica. 2022. PMID: 36534424 Free PMC article. Review.
-
Hereditary Gynecologic Cancer Syndromes - A Narrative Review.Onco Targets Ther. 2022 Apr 8;15:381-405. doi: 10.2147/OTT.S353054. eCollection 2022. Onco Targets Ther. 2022. PMID: 35422633 Free PMC article. Review.
-
Two Genetic Mechanisms in Two Siblings with Intellectual Disability, Autism Spectrum Disorder, and Psychosis.J Pers Med. 2022 Jun 20;12(6):1013. doi: 10.3390/jpm12061013. J Pers Med. 2022. PMID: 35743796 Free PMC article.
-
Molecular pathology of endocrine gland tumors: genetic alterations and clinicopathologic relevance.Virchows Arch. 2024 Feb;484(2):289-319. doi: 10.1007/s00428-023-03713-4. Epub 2023 Dec 18. Virchows Arch. 2024. PMID: 38108848 Free PMC article. Review.
-
Imaging of pituitary tumors: an update with the 5th WHO Classifications-part 2. Neoplasms other than PitNET and tumor-mimicking lesions.Jpn J Radiol. 2023 Aug;41(8):808-829. doi: 10.1007/s11604-023-01407-0. Epub 2023 Mar 13. Jpn J Radiol. 2023. PMID: 36913010 Free PMC article. Review.
References
-
- Denli AM, Tops BB, Plasterk RH, Ketting RF, Hannon GJ. Processing of primary microRNAs by the Microprocessor complex. Nature. 2004;432:231–235. - PubMed
-
- Zhang H, Kolb FA, Jaskiewicz L, Westhof E, Filipowicz W. Single processing center models for human Dicer and bacterial RNase III. Cell. 2004;118:57–68. - PubMed
-
- Bernstein E, Caudy AA, Hammond SM, Hannon GJ. Role for a bidentate ribonuclease in the initiation step of RNA interference. Nature. 2001;409:363–366. - PubMed
-
- Foulkes WD, Priest JR, Duchaine TF. DICER1: mutations, microRNAs and mechanisms. Nat. Rev. Cancer. 2014;14:662–672. - PubMed
-
- Slade I, et al. DICER1 syndrome: clarifying the diagnosis, clinical features and management implications of a pleiotropic tumour predisposition syndrome. J. Med. Genet. 2011;48:273–278. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

