Next Generation Antiobesity Medications: Setmelanotide, Semaglutide, Tirzepatide and Bimagrumab: What do They Mean for Clinical Practice?
- PMID: 34518444
- PMCID: PMC8526285
- DOI: 10.7570/jomes21033
Next Generation Antiobesity Medications: Setmelanotide, Semaglutide, Tirzepatide and Bimagrumab: What do They Mean for Clinical Practice?
Abstract
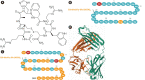
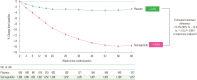
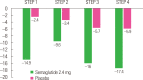
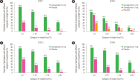
There is a new generation of antiobesity drugs in development or just arriving on the scene. First, setmelanotide has been approved for three of the ultrarare genetic conditions that cause obesity-pro-opiomelanocortin deficiency, proprotein convertase subtilisin and kexin type 1 (an important enzyme in the melanocortin pathway) and leptin receptor deficiency. Setmelanotide marks the first in a personalized medicine approach to obesity. Second, semaglutide 2.4 mg once weekly has been submitted to regulators in the United States and the European Union for approval for patients with obesity (body mass index [BMI] ≥30 kg/m2) or overweight (BMI ≥27 kg/m2) and at least one weight related comorbidity. This drug has been studied in five phase 3 clinical trials, four discussed herein: semaglutide produces roughly twice as much weight loss as we have seen in older antiobesity medications. Semaglutide is already in use for treatment of diabetes and, as a glucagon-like peptide 1 (GLP-1) receptor analog, is part of a class of drugs used widely in diabetes. Tirzepatide, a glucose-insulin peptide and GLP-1 dual agonist is in phase 3 study for obesity management, and bimagrumab is a new agent in phase 2 with a unique mechanism of action; they are generating much interest. The purpose of this narrative review is lay the groundwork for a discussion of the clinical impact of these new medications on the clinical practice of obesity. Further, these developments shall be used to launch a speculation of what is likely to be their impact on the future of obesity pharmacotherapy.
Keywords: Anti-obesity agents; Anti-obesity drugs; Bimagrumab; Semaglutide; Setmelanotide; Tirzepatide; Weight loss agents.
Figures

Similar articles
-
Drugs for Treating Obesity.Handb Exp Pharmacol. 2022;274:387-414. doi: 10.1007/164_2021_560. Handb Exp Pharmacol. 2022. PMID: 34783910
-
Semaglutide as a promising antiobesity drug.Obes Rev. 2019 Jun;20(6):805-815. doi: 10.1111/obr.12839. Epub 2019 Feb 15. Obes Rev. 2019. PMID: 30768766 Review.
-
What is the pipeline for future medications for obesity?Int J Obes (Lond). 2024 Feb 1. doi: 10.1038/s41366-024-01473-y. Online ahead of print. Int J Obes (Lond). 2024. PMID: 38302593 Review.
-
Setmelanotide: a promising advancement for pediatric patients with rare forms of genetic obesity.Curr Opin Endocrinol Diabetes Obes. 2023 Apr 1;30(2):136-140. doi: 10.1097/MED.0000000000000798. Epub 2023 Feb 1. Curr Opin Endocrinol Diabetes Obes. 2023. PMID: 36722447 Free PMC article. Review.
-
Once-Weekly Semaglutide for Weight Management: A Clinical Review.J Pharm Technol. 2022 Aug;38(4):239-246. doi: 10.1177/87551225221092681. Epub 2022 May 13. J Pharm Technol. 2022. PMID: 35832567 Free PMC article. Review.
Cited by
-
G protein-coupled receptors and obesity.Front Endocrinol (Lausanne). 2023 Dec 14;14:1301017. doi: 10.3389/fendo.2023.1301017. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 38161982 Free PMC article. Review.
-
Metabolically Healthy Obesity: Are Interventions Useful?Curr Obes Rep. 2023 Mar;12(1):36-60. doi: 10.1007/s13679-023-00494-4. Epub 2023 Feb 23. Curr Obes Rep. 2023. PMID: 36814043 Review.
-
An Update on Semaglutide Research: A Bibliometric Analysis and a Literature Review.Cureus. 2023 Oct 5;15(10):e46510. doi: 10.7759/cureus.46510. eCollection 2023 Oct. Cureus. 2023. PMID: 37808605 Free PMC article. Review.
-
New Developments in Pharmacological Treatment of Obesity and Type 2 Diabetes-Beyond and within GLP-1 Receptor Agonists.Biomedicines. 2024 Jun 13;12(6):1320. doi: 10.3390/biomedicines12061320. Biomedicines. 2024. PMID: 38927527 Free PMC article. Review.
-
Recent advancements in pharmacological strategies to modulate energy balance for combating obesity.RSC Med Chem. 2023 Jun 14;14(8):1429-1445. doi: 10.1039/d3md00107e. eCollection 2023 Aug 16. RSC Med Chem. 2023. PMID: 37593583 Free PMC article. Review.
References
-
- Kievit P, Halem H, Marks DL, Dong JZ, Glavas MM, Sinnayah P, et al. Chronic treatment with a melanocortin-4 receptor agonist causes weight loss, reduces insulin resistance, and improves cardiovascular function in diet-induced obese rhesus macaques. Diabetes. 2013;62:490–7. doi: 10.2337/db12-0598. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

