The use of germicidal ultraviolet light, vaporised hydrogen peroxide and dry heat to decontaminate face masks and filtering respirators contaminated with an infectious norovirus
- PMID: 34316573
- PMCID: PMC7834285
- DOI: 10.1016/j.infpip.2020.100111
The use of germicidal ultraviolet light, vaporised hydrogen peroxide and dry heat to decontaminate face masks and filtering respirators contaminated with an infectious norovirus
Abstract
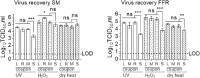
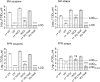
In the context of the SARS-CoV-2 pandemic, reuse of surgical masks and filtering facepiece respirators has been recommended. Their reuse necessitates procedures to inactivate contaminating human respiratory and oral pathogens. We previously demonstrated decontamination of masks and respirators contaminated with an infectious SARS-CoV-2 surrogate via ultraviolet germicidal irradiation, vaporised hydrogen peroxide, and use of dry heat. Here, we show that these same methods efficiently inactivate a more resistant, non-enveloped oral virus; decontamination of infectious murine norovirus-contaminated masks and respirators reduced viral titres by over four orders of magnitude on mask or respirator coupons.
Keywords: Decontamination (UV, H2O2, dry heat); FFR, filtering facepiece respirator; MuNoV, murine norovirus; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SM, surgical mask; norovirus; respirator; surgical mask.
© 2020 The Authors.
Figures
Similar articles
-
The use of germicidal ultraviolet light, vaporized hydrogen peroxide and dry heat to decontaminate face masks and filtering respirators contaminated with a SARS-CoV-2 surrogate virus.J Hosp Infect. 2020 Nov;106(3):577-584. doi: 10.1016/j.jhin.2020.08.025. Epub 2020 Sep 1. J Hosp Infect. 2020. PMID: 32889029 Free PMC article.
-
"Don, doff, discard" to "don, doff, decontaminate"-FFR and mask integrity and inactivation of a SARS-CoV-2 surrogate and a norovirus following multiple vaporised hydrogen peroxide-, ultraviolet germicidal irradiation-, and dry heat decontaminations.PLoS One. 2021 May 19;16(5):e0251872. doi: 10.1371/journal.pone.0251872. eCollection 2021. PLoS One. 2021. PMID: 34010337 Free PMC article.
-
Filtering Facepiece Respirator (N95 Respirator) Reprocessing: A Systematic Review.JAMA. 2021 Apr 6;325(13):1296-1317. doi: 10.1001/jama.2021.2531. JAMA. 2021. PMID: 33656543
-
A Practical Approach to Filtering Facepiece Respirator Decontamination and Reuse: Ultraviolet Germicidal Irradiation.Curr Treat Options Infect Dis. 2021;13(2):35-46. doi: 10.1007/s40506-021-00247-8. Epub 2021 Apr 6. Curr Treat Options Infect Dis. 2021. PMID: 33841050 Free PMC article. Review.
-
Principles and practice for SARS-CoV-2 decontamination of N95 masks with UV-C.Biophys J. 2021 Jul 20;120(14):2927-2942. doi: 10.1016/j.bpj.2021.02.039. Epub 2021 Mar 4. Biophys J. 2021. PMID: 33675766 Free PMC article. Review.
Cited by
-
Indoor air quality improvement in COVID-19 pandemic: Review.Sustain Cities Soc. 2021 Jul;70:102942. doi: 10.1016/j.scs.2021.102942. Epub 2021 Apr 15. Sustain Cities Soc. 2021. PMID: 33889481 Free PMC article.
-
Dry Heat as a Potential Decontamination Method on the Filtration Efficiency of Filtering Facepiece Respirators.Int J Environ Res Public Health. 2022 Jun 11;19(12):7167. doi: 10.3390/ijerph19127167. Int J Environ Res Public Health. 2022. PMID: 35742417 Free PMC article.
-
Noroviruses-The State of the Art, Nearly Fifty Years after Their Initial Discovery.Viruses. 2021 Aug 4;13(8):1541. doi: 10.3390/v13081541. Viruses. 2021. PMID: 34452406 Free PMC article. Review.
-
Of masks and methylene blue-The use of methylene blue photochemical treatment to decontaminate surgical masks contaminated with a tenacious small nonenveloped norovirus.Am J Infect Control. 2022 Aug;50(8):871-877. doi: 10.1016/j.ajic.2022.01.024. Am J Infect Control. 2022. PMID: 35908825 Free PMC article.
-
Exploring the need for surgical face masks in operating room: a comprehensive literature review.Ann Med Surg (Lond). 2024 Sep 5;86(10):6012-6020. doi: 10.1097/MS9.0000000000002542. eCollection 2024 Oct. Ann Med Surg (Lond). 2024. PMID: 39359805 Free PMC article. Review.
References
-
- World Health Organization (WHO) vol. 2019. WHO; 2020. pp. 1–7. (Rational use of personal protective equipment for coronavirus disease 2019 (COVID-19)).
-
- Center for Devices and Radiological Health . 2020. Enforcement policy for face masks and respirators during the coronavirus disease (COVID-19) public health emergency (revised) guidance for industry and food and drug administration staff.
LinkOut - more resources
Full Text Sources
Miscellaneous

