PlzA is a bifunctional c-di-GMP biosensor that promotes tick and mammalian host-adaptation of Borrelia burgdorferi
- PMID: 34265024
- PMCID: PMC8323883
- DOI: 10.1371/journal.ppat.1009725
PlzA is a bifunctional c-di-GMP biosensor that promotes tick and mammalian host-adaptation of Borrelia burgdorferi
Abstract
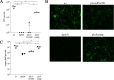
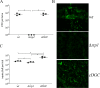
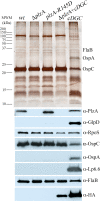
In this study, we examined the relationship between c-di-GMP and its only known effector protein, PlzA, in Borrelia burgdorferi during the arthropod and mammalian phases of the enzootic cycle. Using a B. burgdorferi strain expressing a plzA point mutant (plzA-R145D) unable to bind c-di-GMP, we confirmed that the protective function of PlzA in ticks is c-di-GMP-dependent. Unlike ΔplzA spirochetes, which are severely attenuated in mice, the plzA-R145D strain was fully infectious, firmly establishing that PlzA serves a c-di-GMP-independent function in mammals. Contrary to prior reports, loss of PlzA did not affect expression of RpoS or RpoS-dependent genes, which are essential for transmission, mammalian host-adaptation and murine infection. To ascertain the nature of PlzA's c-di-GMP-independent function(s), we employed infection models using (i) host-adapted mutant spirochetes for needle inoculation of immunocompetent mice and (ii) infection of scid mice with in vitro-grown organisms. Both approaches substantially restored ΔplzA infectivity, suggesting that PlzA enables B. burgdorferi to overcome an early bottleneck to infection. Furthermore, using a Borrelia strain expressing a heterologous, constitutively active diguanylate cyclase, we demonstrate that 'ectopic' production of c-di-GMP in mammals abrogates spirochete virulence and interferes with RpoS function at the post-translational level in a PlzA-dependent manner. Structural modeling and SAXS analysis of liganded- and unliganded-PlzA revealed marked conformational changes that underlie its biphasic functionality. This structural plasticity likely enables PlzA to serve as a c-di-GMP biosensor that in its respective liganded and unliganded states promote vector- and host-adaptation by the Lyme disease spirochete.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



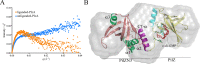
Similar articles
-
The Borrelia burgdorferi c-di-GMP Binding Receptors, PlzA and PlzB, Are Functionally Distinct.Front Cell Infect Microbiol. 2018 Jul 11;8:213. doi: 10.3389/fcimb.2018.00213. eCollection 2018. Front Cell Infect Microbiol. 2018. PMID: 30050868 Free PMC article.
-
Positive and Negative Regulation of Glycerol Utilization by the c-di-GMP Binding Protein PlzA in Borrelia burgdorferi.J Bacteriol. 2018 Oct 23;200(22):e00243-18. doi: 10.1128/JB.00243-18. Print 2018 Nov 15. J Bacteriol. 2018. PMID: 30181123 Free PMC article.
-
Cyclic Di-GMP receptor PlzA controls virulence gene expression through RpoS in Borrelia burgdorferi.Infect Immun. 2014 Jan;82(1):445-52. doi: 10.1128/IAI.01238-13. Epub 2013 Nov 11. Infect Immun. 2014. PMID: 24218478 Free PMC article.
-
The cyclic-di-GMP signaling pathway in the Lyme disease spirochete, Borrelia burgdorferi.Front Cell Infect Microbiol. 2014 May 1;4:56. doi: 10.3389/fcimb.2014.00056. eCollection 2014. Front Cell Infect Microbiol. 2014. PMID: 24822172 Free PMC article. Review.
-
Interaction of the Lyme disease spirochete with its tick vector.Cell Microbiol. 2016 Jul;18(7):919-27. doi: 10.1111/cmi.12609. Epub 2016 May 24. Cell Microbiol. 2016. PMID: 27147446 Free PMC article. Review.
Cited by
-
BosR and PlzA reciprocally regulate RpoS function to sustain Borrelia burgdorferi in ticks and mammals.J Clin Invest. 2023 Mar 1;133(5):e166710. doi: 10.1172/JCI166710. J Clin Invest. 2023. PMID: 36649080 Free PMC article.
-
Potential Regulatory Role in Mammalian Host Adaptation for a Small Intergenic Region of Lp17 in the Lyme Disease Spirochete.Front Cell Infect Microbiol. 2022 May 2;12:892220. doi: 10.3389/fcimb.2022.892220. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 35586252 Free PMC article.
-
Borrelia burgdorferi PlzA is a cyclic-di-GMP dependent DNA and RNA binding protein.bioRxiv [Preprint]. 2024 Jan 22:2023.01.30.526351. doi: 10.1101/2023.01.30.526351. bioRxiv. 2024. Update in: Mol Microbiol. 2024 May;121(5):1039-1062. doi: 10.1111/mmi.15254. PMID: 36778503 Free PMC article. Updated. Preprint.
-
c-di-GMP regulates activity of the PlzA RNA chaperone from the Lyme disease spirochete.Mol Microbiol. 2023 Jun;119(6):711-727. doi: 10.1111/mmi.15066. Epub 2023 Apr 21. Mol Microbiol. 2023. PMID: 37086029 Free PMC article.
-
Pathogenicity and virulence of Borrelia burgdorferi.Virulence. 2023 Dec;14(1):2265015. doi: 10.1080/21505594.2023.2265015. Epub 2023 Oct 9. Virulence. 2023. PMID: 37814488 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

