[Simultaneous determination of 18 chlorinated hydrocarbon organic solvents in cosmetics by gas chromatography-mass spectrometry]
- PMID: 34227313
- PMCID: PMC9403812
- DOI: 10.3724/SP.J.1123.2020.05010
[Simultaneous determination of 18 chlorinated hydrocarbon organic solvents in cosmetics by gas chromatography-mass spectrometry]
Abstract
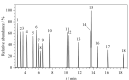
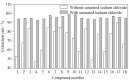
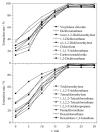
Organic solvents can be used to dissolve and disperse flavors, bactericides, preservatives, surfactants, oils, and coloring agents during the production of cosmetics. However, harmful chlorinated hydrocarbon organic solvents are found in cosmetics such as manicure products, anti-acne products, and perfumes. Long-term contact with such cosmetics will have an adverse effect on the consumers' health. Past research has focused on very few chlorinated hydrocarbon organic solvents in cosmetics. Most organic solvents with low boiling points are typically determined by headspace-gas chromatography-mass spectrometry. In this study, a high-boiling-point solvent was used as the injection solvent, and the solvent delay time was cancelled. The compounds that could only peak during the solvent delay time were effectively separated. A method coupling sample pretreatment with gas chromatography-mass spectrometry (GC-MS) was developed for the simultaneous determination of 18 chlorinated hydrocarbon organic solvents in cosmetics: vinylidene chloride, dichloromethane, trans-1,2-dichloroethylene, 1,1-dichloroethane, cis-1,2-dichloroethylene, chloroform, 1,1,1-trichloroethane, carbon tetrachloride, 1,2-dichloroethane, trichloroethylene, 1,1,2-trichloroethane, tetrachloroethylene, 1,1,1,2-tetrachloroethane, 1,1,2,2-tetrachloroethane, 1,2,3-trichloropropane, pentachloroethane, hexachloroethane, and hexachloro-1,3-butadiene. These 18 solvents have a wide range of polarities and a notable difference in volatilities, in addition to many isomers and structural analogs, which renders their separation difficult. Therefore, the separation effect of three kinds of GC columns with different polarities was compared. n-Tetradecane, an injection solvent with good solubility, was selected as the extraction solvent. An organic solvent with high polarity has low extraction rate because of its weak polarity. Adding sodium chloride solution to the sample to induce the "salting out" effect could change the partition coefficient of the components, thereby improving the extraction rate. Therefore, the concentration of the sodium chloride solution added to the sample was optimized. In this work, liquid-liquid extraction was the main extraction process, so the effects of different shaking times, temperatures, and frequencies on the extraction rate were discussed. The optimized results are as follows: at normal temperature, the sample dispersed or dissolved in saturated sodium chloride solution was extracted by n-tetradecane at an oscillating speed of 100 r/min for 20 min. Separation was performed on an Agilent J&W DB-624 column (30 m×0.25 mm×1.4 μm) by GC-MS with an electrospray ionization (EI) source in the selected ion monitoring (SIM) mode. The external standard method was used for quantitative determination. The 18 compounds could be analyzed within 19 min. The linear equations, linear correlation coefficients, and linear ranges were obtained by analyzing a series of mixed standard working solutions. The limits of detection (LODs, S/N=3) and limits of quantification (LOQs, S/N=10) of the 18 components were determined. The negative lipstick (solid) and mouthwash (liquid) samples were used as the spiked sample matrix at three levels, and the recoveries and precisions were calculated. The calibration curves showed good linearities for the 18 chlorinated hydrocarbon organic solvents in range of 0.2-100 mg/L, with correlation coefficients (R2) not less than 0.9992. The LODs and LOQs were in the range of 0.033-0.049 mg/L and 0.10-0.15 mg/L, respectively. The average recoveries of the 18 chlorinated hydrocarbon organic solvents in lipstick (solid) and mouthwash (liquid) were 92.4%-103.1% and 93.3%-102.4% respectively; the corresponding relative standard deviations (RSDs) were 3.1%-5.3% and 2.8%-5.4% (n=6). This method was used to determine 115 different types of cosmetics, and tetrachloroethylene was detected in three nail polishes. With its advantages of high sensitivity, good precision, and accuracy, the developed method is suitable for the quantitative analysis of the aforesaid 18 compounds in all kinds of cosmetics. The study findings would serve as a reference for the quality and safety monitoring of cosmetics.
建立了同时测定化妆品中18种氯代烃类有机溶剂的气相色谱-质谱(GC-MS)检测方法。样品在饱和氯化钠溶液中由正十四烷振荡提取后,以Agilent J&W DB-624超高惰性毛细管柱(30 m×0.25 mm×1.4 μm)为分离色谱柱进行分析,以电子轰击(EI)源、SIM模式进行质谱监测,外标法定量。结果表明,18种化合物在19 min内完成色谱分离分析,检出限(LOD, S/N=3)和定量限(LOQ, S/N=10)分别为0.033~0.049 mg/L和0.10~0.15 mg/L, 18种氯代烃类有机溶剂在0.2~100 mg/L线性范围内线性关系良好,相关系数(R2)均不小于0.9992。以阴性样品口红(固体)和漱口水(液体)为样品基质,在不同添加水平下,18种氯代烃类有机溶剂的平均回收率分别为92.4%~103.1%和93.3%~102.4%,相对标准偏差(RSD, n=6)分别为3.1%~5.3%和2.8%~5.4%。采用该方法对115个化妆品样品进行测定,3个指甲油样品均检测出四氯乙烯,测定值为11.4~42.0 g/kg。研究建立的方法采用高沸点溶剂作为进样溶剂,取消溶剂延迟时间,使只能在溶剂延迟时间出峰的化合物得到有效色谱分离,分析时间短,且重复性好,灵敏度高,可同时检测各种化妆品中多种氯代烃类有机溶剂。该方法的建立为我国化妆品中氯代烃类有机溶剂检测标准的制订和质量安全监控提供了参考。
Keywords: chlorinated hydrocarbon organic solvents; cosmetics; gas chromatography-mass spectrometry (GC-MS).
Figures
Similar articles
-
[Determination of 13 sunscreen agents in cosmetics by gas chromatography-mass spectrometry].Se Pu. 2021 May;39(5):552-557. doi: 10.3724/SP.J.1123.2020.11003. Se Pu. 2021. PMID: 34227340 Free PMC article. Chinese.
-
[Simultaneous determination of 10 quaternary ammonium salt bactericides in oral care products by high performance liquid chromatography-evaporative light-scattering detection].Se Pu. 2024 Aug;42(8):783-791. doi: 10.3724/SP.J.1123.2023.10013. Se Pu. 2024. PMID: 39086247 Free PMC article. Chinese.
-
[Simultaneous determination of seven dimethylcyclosiloxanes in cosmetics of different formulation systems by gel permeation chromatography purification-gas chromatography-tandem mass spectrometry].Se Pu. 2022 Jun;40(6):576-583. doi: 10.3724/SP.J.1123.2021.11024. Se Pu. 2022. PMID: 35616203 Free PMC article. Chinese.
-
[Advances in the development of detection techniques for organophosphate ester flame retardants in food].Se Pu. 2020 Dec 8;38(12):1369-1380. doi: 10.3724/SP.J.1123.2020.03026. Se Pu. 2020. PMID: 34213251 Review. Chinese.
-
Residual Organic Solvent Analysis in Nanoformulations Using Headspace Gas Chromatography: Version 1.2022 Sep. In: National Cancer Institute’s Nanotechnology Characterization Laboratory Assay Cascade Protocols [Internet]. Bethesda (MD): National Cancer Institute (US); 2005 May 1–. NCL Method PCC-22. 2022 Sep. In: National Cancer Institute’s Nanotechnology Characterization Laboratory Assay Cascade Protocols [Internet]. Bethesda (MD): National Cancer Institute (US); 2005 May 1–. NCL Method PCC-22. PMID: 39012987 Free Books & Documents. Review.
References
-
- Wang M L, Fang S, Liang X R. J Pharm Biomed Anal, 2018,158(5):262 - PubMed
-
- José Ángel S D, Soledad G R, Diego G G, et al.. Anal Chim Acta, 2019,1046(10):132 - PubMed
-
- Nacham O, Ho T D, Anderson J L, et al.. J Pharm Biomed Anal, 2017,145(25):879 - PubMed
-
- Yu Y J, Chen B, Shen C D, et al.. J Chromatogr A, 2010,1217(32):5158 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous