Lipase-Catalyzed Production of Sorbitol Laurate in a "2-in-1" Deep Eutectic System: Factors Affecting the Synthesis and Scalability
- PMID: 34067126
- PMCID: PMC8124474
- DOI: 10.3390/molecules26092759
Lipase-Catalyzed Production of Sorbitol Laurate in a "2-in-1" Deep Eutectic System: Factors Affecting the Synthesis and Scalability
Abstract
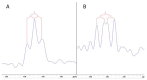
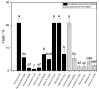
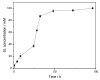
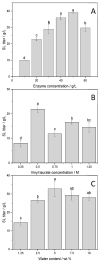
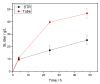
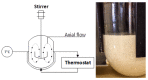
Surfactants, such as glycolipids, are specialty compounds that can be encountered daily in cleaning agents, pharmaceuticals or even in food. Due to their wide range of applications and, more notably, their presence in hygiene products, the demand is continuously increasing worldwide. The established chemical synthesis of glycolipids presents several disadvantages, such as lack of specificity and selectivity. Moreover, the solubility of polyols, such as sugars or sugar alcohols, in organic solvents is rather low. The enzymatic synthesis of these compounds is, however, possible in nearly water-free media using inexpensive and renewable building blocks. Using lipases, ester formation can be achieved under mild conditions. We propose, herein, a "2-in-1" system that overcomes solubility problems, as a Deep Eutectic System (DES) made of sorbitol and choline chloride replaces either a purely organic or aqueous medium. For the first time, 16 commercially available lipase formulations were compared, and the factors affecting the conversion were investigated to optimize this process, owing to a newly developed High-Performance Liquid Chromatography-Evaporative Light Scattering Detector (HPLC-ELSD) method for quantification. Thus, using 50 g/L of lipase formulation Novozym 435® at 50 °C, the optimized synthesis of sorbitol laurate (SL) allowed to achieve 28% molar conversion of 0.5 M of vinyl laurate to its sugar alcohol monoester when the DES contained 5 wt.% water. After 48h, the de novo synthesized glycolipid was separated from the media by liquid-liquid extraction, purified by flash-chromatography and characterized thoroughly by one- and two-dimensional Nuclear Magnetic Resonance (NMR) experiments combined to Mass Spectrometry (MS). In completion, we provide initial proof of scalability for this process. Using a 2.5 L stirred tank reactor (STR) allowed a batch production reaching 25 g/L in a highly viscous two-phase system.
Keywords: biosynthesis; ester; glycolipid; optimization; sugar alcohol; unconventional media.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
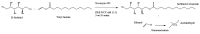
Similar articles
-
Microwave-Assisted One-Pot Lipid Extraction and Glycolipid Production from Oleaginous Yeast Saitozyma podzolica in Sugar Alcohol-Based Media.Molecules. 2021 Jan 18;26(2):470. doi: 10.3390/molecules26020470. Molecules. 2021. PMID: 33477445 Free PMC article.
-
Deep Eutectic Solvents as Suitable Solvents for Lipase-Catalyzed Transesterification Reactions.ChemSusChem. 2023 Oct 20;16(20):e202300615. doi: 10.1002/cssc.202300615. Epub 2023 Aug 9. ChemSusChem. 2023. PMID: 37423894
-
Lipase-Catalyzed Synthesis of Sugar Esters in Honey and Agave Syrup.Front Chem. 2018 Feb 12;6:24. doi: 10.3389/fchem.2018.00024. eCollection 2018. Front Chem. 2018. PMID: 29487847 Free PMC article.
-
Parameters Influencing Lipase-Catalyzed Glycolipid Synthesis by (Trans-)Esterification Reaction.Adv Biochem Eng Biotechnol. 2022;181:53-72. doi: 10.1007/10_2021_173. Adv Biochem Eng Biotechnol. 2022. PMID: 34518911 Review.
-
Forced degradation and impurity profiling: recent trends in analytical perspectives.J Pharm Biomed Anal. 2013 Dec;86:11-35. doi: 10.1016/j.jpba.2013.07.013. Epub 2013 Jul 31. J Pharm Biomed Anal. 2013. PMID: 23969330 Review.
Cited by
-
Esters in the Food and Cosmetic Industries: An Overview of the Reactors Used in Their Biocatalytic Synthesis.Materials (Basel). 2024 Jan 4;17(1):268. doi: 10.3390/ma17010268. Materials (Basel). 2024. PMID: 38204120 Free PMC article. Review.
-
Combination of Enzymes and Deep Eutectic Solvents as Powerful Toolbox for Organic Synthesis.Molecules. 2023 Jan 5;28(2):516. doi: 10.3390/molecules28020516. Molecules. 2023. PMID: 36677575 Free PMC article. Review.
-
Enhanced Bioactivity of Tailor-Made Glycolipid Enriched Manuka Honey.Int J Mol Sci. 2022 Oct 10;23(19):12031. doi: 10.3390/ijms231912031. Int J Mol Sci. 2022. PMID: 36233331 Free PMC article.
-
Intensification of Enzymatic Sorbityl Laurate Production in Dissolved and Neat Systems under Conventional and Microwave Heating.ACS Omega. 2024 Apr 1;9(15):17163-17173. doi: 10.1021/acsomega.3c10004. eCollection 2024 Apr 16. ACS Omega. 2024. PMID: 38645351 Free PMC article.
-
Lipase-Mediated Mechanoenzymatic Synthesis of Sugar Esters in Dissolved Unconventional and Neat Reaction Systems.ACS Sustain Chem Eng. 2022 Aug 8;10(31):10192-10202. doi: 10.1021/acssuschemeng.2c01727. Epub 2022 Jul 27. ACS Sustain Chem Eng. 2022. PMID: 35966390 Free PMC article.
References
-
- Selmair P.L., Koehler P. Role of glycolipids in breadmaking. Lipid Technol. 2010;22:7–10. doi: 10.1002/lite.200900069. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

