Effects of Resmetirom on Noninvasive Endpoints in a 36-Week Phase 2 Active Treatment Extension Study in Patients With NASH
- PMID: 33860116
- PMCID: PMC8034581
- DOI: 10.1002/hep4.1657
Effects of Resmetirom on Noninvasive Endpoints in a 36-Week Phase 2 Active Treatment Extension Study in Patients With NASH
Abstract
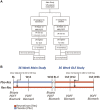
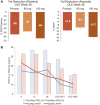
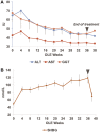
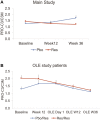
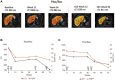
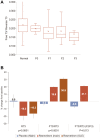
Resmetirom (MGL-3196), a selective thyroid hormone receptor-β agonist, was evaluated in a 36-week paired liver biopsy study (NCT02912260) in adults with biopsy-confirmed nonalcoholic steatohepatitis (NASH). The primary endpoint was relative liver fat reduction as assessed by MRI-proton density fat fraction (MRI-PDFF), and secondary endpoints included histopathology. Subsequently, a 36-week active treatment open-label extension (OLE) study was conducted in 31 consenting patients (including 14 former placebo patients) with persistently mild to markedly elevated liver enzymes at the end of the main study. In patients treated with resmetirom (80 or 100 mg orally per day), MRI-PDFF reduction at OLE week 36 was -11.1% (1.5%) mean reduction (standard error [SE]; P < 0.0001) and -52.3% (4.4%) mean relative reduction, P < 0.0001. Low-density lipoprotein (LDL) cholesterol (-26.1% [4.5%], P < 0.0001), apolipoprotein B (-23.8% [3.0%], P < 0.0001), and triglycerides (-19.6% [5.4%], P = 0.0012; -46.1 [14.5] mg/dL, P = 0.0031) were reduced from baseline. Markers of fibrosis were reduced, including liver stiffness assessed by transient elastography (-2.1 [0.8] mean kilopascals [SE], P = 0.015) and N-terminal type III collagen pro-peptide (PRO-C3) (-9.8 [2.3] ng/mL, P = 0.0004 (baseline ≥ 10 ng/mL). In the main and OLE studies, PRO-C3/C3M (matrix metalloproteinase-degraded C3), a marker of net fibrosis formation, was reduced in resmetirom-treated patients (-0.76 [-1.27, -0.24], P = 0.0044 and -0.68, P < 0.0001, respectively). Resmetirom was well tolerated, with few, nonserious adverse events. Conclusion: The results of this 36-week OLE study support the efficacy and safety of resmetirom at daily doses of 80 mg and 100 mg, used in the ongoing phase 3 NASH study, MAESTRO-NASH (NCT03900429). The OLE study demonstrates a potential for noninvasive assessments to monitor the response to resmetirom from an individual patient with NASH.
© 2021 The Authors. Hepatology Communications published by Wiley Periodicals LLC on behalf of the American Association for the Study of Liver Diseases.
Figures
Similar articles
-
Resmetirom: A Systematic Review of the Revolutionizing Approach to Non-alcoholic Steatohepatitis Treatment Focusing on Efficacy, Safety, Cost-Effectiveness, and Impact on Quality of Life.Cureus. 2024 Sep 22;16(9):e69919. doi: 10.7759/cureus.69919. eCollection 2024 Sep. Cureus. 2024. PMID: 39439647 Free PMC article. Review.
-
Resmetirom (MGL-3196) for the treatment of non-alcoholic steatohepatitis: a multicentre, randomised, double-blind, placebo-controlled, phase 2 trial.Lancet. 2019 Nov 30;394(10213):2012-2024. doi: 10.1016/S0140-6736(19)32517-6. Epub 2019 Nov 11. Lancet. 2019. PMID: 31727409 Clinical Trial.
-
A Phase 3, Randomized, Controlled Trial of Resmetirom in NASH with Liver Fibrosis.N Engl J Med. 2024 Feb 8;390(6):497-509. doi: 10.1056/NEJMoa2309000. N Engl J Med. 2024. PMID: 38324483 Clinical Trial.
-
Design of the phase 3 MAESTRO clinical program to evaluate resmetirom for the treatment of nonalcoholic steatohepatitis.Aliment Pharmacol Ther. 2024 Jan;59(1):51-63. doi: 10.1111/apt.17734. Epub 2023 Oct 2. Aliment Pharmacol Ther. 2024. PMID: 37786277 Clinical Trial.
-
Resmetirom: An Orally Administered, Smallmolecule, Liver-directed, β-selective THR Agonist for the Treatment of Non-alcoholic Fatty Liver Disease and Non-alcoholic Steatohepatitis.touchREV Endocrinol. 2023 May;19(1):60-70. doi: 10.17925/EE.2023.19.1.60. Epub 2023 May 1. touchREV Endocrinol. 2023. PMID: 37313239 Free PMC article. Review.
Cited by
-
Resmetirom: A Systematic Review of the Revolutionizing Approach to Non-alcoholic Steatohepatitis Treatment Focusing on Efficacy, Safety, Cost-Effectiveness, and Impact on Quality of Life.Cureus. 2024 Sep 22;16(9):e69919. doi: 10.7759/cureus.69919. eCollection 2024 Sep. Cureus. 2024. PMID: 39439647 Free PMC article. Review.
-
Lessons on Drug Development: A Literature Review of Challenges Faced in Nonalcoholic Fatty Liver Disease (NAFLD) Clinical Trials.Int J Mol Sci. 2022 Dec 21;24(1):158. doi: 10.3390/ijms24010158. Int J Mol Sci. 2022. PMID: 36613602 Free PMC article. Review.
-
Bile acid and receptors: biology and drug discovery for nonalcoholic fatty liver disease.Acta Pharmacol Sin. 2022 May;43(5):1103-1119. doi: 10.1038/s41401-022-00880-z. Epub 2022 Feb 25. Acta Pharmacol Sin. 2022. PMID: 35217817 Free PMC article. Review.
-
Potential Therapeutic Targets and Promising Agents for Combating NAFLD.Biomedicines. 2022 Apr 14;10(4):901. doi: 10.3390/biomedicines10040901. Biomedicines. 2022. PMID: 35453652 Free PMC article. Review.
-
TG68, a Novel Thyroid Hormone Receptor-β Agonist for the Treatment of NAFLD.Int J Mol Sci. 2021 Dec 3;22(23):13105. doi: 10.3390/ijms222313105. Int J Mol Sci. 2021. PMID: 34884910 Free PMC article.
References
-
- Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2018;67:328‐357. - PubMed
-
- Diehl AM, Day C. Cause, pathogenesis, and treatment of nonalcoholic steatohepatitis. N Engl J Med 2017;377:2063‐2072. - PubMed
-
- Stahl EP, Dhindsa DS, Lee SK, Sandesara PB, Chalasani NP, Sperling LS. Nonalcoholic fatty liver disease and the heart: JACC state‐of‐the‐art review. J Am Coll Cardiol 2019;73:948‐963. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

