Alpha-1 antitrypsin (AAT) augmentation therapy in individuals with the PI*MZ genotype: a pro/con debate on a working hypothesis
- PMID: 33757485
- PMCID: PMC7989144
- DOI: 10.1186/s12890-021-01466-x
Alpha-1 antitrypsin (AAT) augmentation therapy in individuals with the PI*MZ genotype: a pro/con debate on a working hypothesis
Abstract
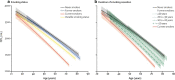
Alpha-1 antitrypsin deficiency (AATD) is a significantly under-diagnosed genetic condition caused by reduced levels and/or functionality of alpha-1 antitrypsin (AAT), predisposing individuals to lung, liver or other systemic diseases. The management of individuals with the PI*MZ genotype, characterized by mild or moderate AAT deficiency, is less clear than of those with the most common severe deficiency genotype (PI*ZZ). Recent genetic data suggest that the PI*MZ genotype may be significantly more prevalent than currently thought. The only specific treatment for lung disease associated with severe AATD is the intravenous infusion of AAT augmentation therapy, which has been shown to slow disease progression in PI*ZZ individuals. There is no specific evidence for the clinical benefit of AAT therapy in PI*MZ individuals, and the risk of emphysema development in this group remains controversial. As such, current guidelines do not support the use of AAT augmentation in PI*MZ individuals. Here, we discuss the limited data on the PI*MZ genotype and offer pro and con perspectives on pursuing an AAT-specific therapeutic strategy in PI*MZ individuals with lung disease. Ultimately, further research to demonstrate the safety, risk/benefit balance and efficacy of AAT therapy in PI*MZ individuals is needed.
Keywords: Alpha-1 antitrypsin deficiency; Chronic obstructive pulmonary disease; Genotype; PI*MZ; Pulmonary disease.
Conflict of interest statement
IB has received consulting fees from Astra Zeneca, Boehringer Ingelheim, CSL Behring, Grifols, Verona Pharma, GE Healthcare, Mylan, Theravance, GSK and has received research grants from AMGEN, GE Healthcare, Theravance and Mylan. MM has received speaker fees from AstraZeneca, Boehringer Ingelheim, Chiesi, Cipla, Menarini, Rovi, Bial, Sandoz, Zambon, CSL Behring, Grifols and Novartis, consulting fees from AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Bial, Gebro Pharma, Kamada, CSL Behring, Laboratorios Esteve, Ferrer, Mereo Biopharma, Verona Pharma, TEVA, Spin Therapeutics, pH Pharma, Novartis, Sanofi and Grifols and research grants from GlaxoSmithKline and Grifols.
Figures
Similar articles
-
Indications for active case searches and intravenous alpha-1 antitrypsin treatment for patients with alpha-1 antitrypsin deficiency chronic pulmonary obstructive disease: an update.Arch Bronconeumol. 2015 Apr;51(4):185-92. doi: 10.1016/j.arbres.2014.05.008. Epub 2014 Jul 12. Arch Bronconeumol. 2015. PMID: 25027067 English, Spanish.
-
Comparison among populations with severe and intermediate alpha1-antitrypsin deficiency and chronic obstructive pulmonary disease.Minerva Med. 2024 Feb;115(1):23-31. doi: 10.23736/S0026-4806.22.08266-0. Epub 2023 Apr 6. Minerva Med. 2024. PMID: 37021471
-
Liver Phenotypes of European Adults Heterozygous or Homozygous for Pi∗Z Variant of AAT (Pi∗MZ vs Pi∗ZZ genotype) and Noncarriers.Gastroenterology. 2020 Aug;159(2):534-548.e11. doi: 10.1053/j.gastro.2020.04.058. Epub 2020 May 4. Gastroenterology. 2020. PMID: 32376409
-
Long-term clinical outcomes following treatment with alpha 1-proteinase inhibitor for COPD associated with alpha-1 antitrypsin deficiency: a look at the evidence.Respir Res. 2017 May 30;18(1):105. doi: 10.1186/s12931-017-0574-1. Respir Res. 2017. PMID: 28558837 Free PMC article. Review.
-
alpha1-Antitrypsin augmentation therapy for PI*MZ heterozygotes: a cautionary note.Chest. 2008 Oct;134(4):831-834. doi: 10.1378/chest.08-0868. Chest. 2008. PMID: 18842915 Review.
Cited by
-
Personalised indication of augmentation therapy for emphysema associated with severe alpha-1 antitrypsin deficiency: a case series.Ther Adv Respir Dis. 2024 Jan-Dec;18:17534666241271917. doi: 10.1177/17534666241271917. Ther Adv Respir Dis. 2024. PMID: 39132722 Free PMC article.
-
Recombinant Alpha-1 Antitrypsin-Fc Fusion Protein INBRX-101 in Adults With Alpha-1 Antitrypsin Deficiency: A Phase 1 Study.Chronic Obstr Pulm Dis. 2024 May 29;11(3):282-292. doi: 10.15326/jcopdf.2023.0469. Chronic Obstr Pulm Dis. 2024. PMID: 38809792 Free PMC article.
-
Transplant with MZ genotype liver: what is the clinical pulmonary picture after 30 years? a case report and review of the literature.J Med Case Rep. 2023 Nov 2;17(1):475. doi: 10.1186/s13256-023-04183-7. J Med Case Rep. 2023. PMID: 37915102 Free PMC article. Review.
-
Nine controversial questions about augmentation therapy for alpha-1 antitrypsin deficiency: a viewpoint.Eur Respir Rev. 2023 Dec 6;32(170):230170. doi: 10.1183/16000617.0170-2023. Print 2023 Dec 31. Eur Respir Rev. 2023. PMID: 38056890 Free PMC article.
-
Estimated Prevalence and Number of PiMZ Genotypes of Alpha-1 Antitrypsin in Seventy-Four Countries Worldwide.Int J Chron Obstruct Pulmon Dis. 2021 Sep 17;16:2617-2630. doi: 10.2147/COPD.S327803. eCollection 2021. Int J Chron Obstruct Pulmon Dis. 2021. PMID: 34556982 Free PMC article. Review.
References
-
- de Serres FJ, Blanco I. Prevalence of alpha1-antitrypsin deficiency alleles PI*S and PI*Z worldwide and effective screening for each of the five phenotypic classes PI*MS, PI*MZ, PI*SS, PI*SZ, and PI*ZZ: a comprehensive review. Ther Adv Respir Dis. 2012;6(5):277–295. doi: 10.1177/1753465812457113. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

