PD-1 and PD-L1 expression on TILs in peritoneal metastases compared to ovarian tumor tissues and its associations with clinical outcome
- PMID: 33737722
- PMCID: PMC7973418
- DOI: 10.1038/s41598-021-85966-0
PD-1 and PD-L1 expression on TILs in peritoneal metastases compared to ovarian tumor tissues and its associations with clinical outcome
Abstract
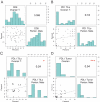
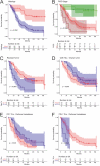
The therapeutic potential of immune checkpoint inhibitors is currently being investigated in epithelial ovarian cancer (EOC), but immunological effects of the programmed cell death protein 1 (PD-1)/programmed cell death 1 ligand 1 (PD-L1) axis in EOC still remain poorly understood. The aim of this study was thus to compare infiltration rates of PD-1 and PD-L1 expressing tumor infiltrating leucocytes (TILs) in primary ovarian tumor tissue and metastatic intraperitoneal implants and to investigate its impact on overall survival (OS). Tumor specimens (ovarian tumor tissues and intraperitoneal metastases) of 111 patients were used to investigate the PD-1, PD-L1 and CD8 expression rates on TILs and PD-L1 expression rate of tumor cells. The percentages of CD8, PD-1, and PD-L1 expressing subpopulations of TILs differ in primary ovarian tumor tissues and metastatic intraperitoneal implants. High PD-1 among TILs in peritoneal metastases were associated with favorable OS. High PD-L1 expression in TILs was associated with poor OS. Combining both factors in peritoneal metastases revealed an unfavorable prognosis. Primary ovarian tumor tissue and intraperitoneal metastatic tissues in EOC might have different strategies to evade immune control. Those findings are of importance for the process of biomarker assessment to predict patients' response to immunotherapy.
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Neoadjuvant chemotherapy (NACT) increases immune infiltration and programmed death-ligand 1 (PD-L1) expression in epithelial ovarian cancer (EOC).Ann Oncol. 2017 Mar 1;28(3):651-657. doi: 10.1093/annonc/mdw625. Ann Oncol. 2017. PMID: 27864219
-
Programmed death ligand-1 and CD8 tumor-infiltrating lymphocytes (TILs) as prognostic predictors in ovarian high-grade serous carcinoma (HGSC).J Egypt Natl Canc Inst. 2021 Jul 9;33(1):16. doi: 10.1186/s43046-021-00073-5. J Egypt Natl Canc Inst. 2021. PMID: 34241710
-
Prognostic impact of programmed cell death-1 (PD-1) and PD-ligand 1 (PD-L1) expression in cancer cells and tumor-infiltrating lymphocytes in ovarian high grade serous carcinoma.Oncotarget. 2016 Jan 12;7(2):1486-99. doi: 10.18632/oncotarget.6429. Oncotarget. 2016. PMID: 26625204 Free PMC article.
-
Significance of evaluating tumor-infiltrating lymphocytes (TILs) and programmed cell death-ligand 1 (PD-L1) expression in breast cancer.Med Mol Morphol. 2017 Dec;50(4):185-194. doi: 10.1007/s00795-017-0170-y. Epub 2017 Sep 21. Med Mol Morphol. 2017. PMID: 28936553 Review.
-
The Impact of Neoadjuvant Chemotherapy on Ovarian Cancer Tumor Microenvironment: A Systematic Review of the Literature.Int J Mol Sci. 2024 Jun 27;25(13):7070. doi: 10.3390/ijms25137070. Int J Mol Sci. 2024. PMID: 39000178 Free PMC article. Review.
Cited by
-
Tumor-Infiltrating Lymphocytes (TILs) in Epithelial Ovarian Cancer: Heterogeneity, Prognostic Impact, and Relationship with Immune Checkpoints.Cancers (Basel). 2022 Oct 29;14(21):5332. doi: 10.3390/cancers14215332. Cancers (Basel). 2022. PMID: 36358750 Free PMC article. Review.
-
Estrogens and the Schrödinger's Cat in the Ovarian Tumor Microenvironment.Cancers (Basel). 2021 Oct 6;13(19):5011. doi: 10.3390/cancers13195011. Cancers (Basel). 2021. PMID: 34638494 Free PMC article. Review.
-
Associations between DNA Damage and PD-L1 Expression in Ovarian Cancer, a Potential Biomarker for Clinical Response.Biology (Basel). 2021 Apr 29;10(5):385. doi: 10.3390/biology10050385. Biology (Basel). 2021. PMID: 33946684 Free PMC article.
-
PD-L1 Expression in Different Segments and Histological Types of Ovarian Cancer According to Lymphocytic Infiltrate.Medicina (Kaunas). 2021 Nov 29;57(12):1309. doi: 10.3390/medicina57121309. Medicina (Kaunas). 2021. PMID: 34946254 Free PMC article.
-
PD-L1 Expression on Circulating Tumour-Derived Microvesicles as a Complementary Tool for Stratification of High-Grade Serous Ovarian Cancer Patients.Cancers (Basel). 2021 Oct 16;13(20):5200. doi: 10.3390/cancers13205200. Cancers (Basel). 2021. PMID: 34680346 Free PMC article.
References
-
- Rizvi NA, et al. Activity and safety of nivolumab, an anti-PD-1 immune checkpoint inhibitor, for patients with advanced, refractory squamous non-small-cell lung cancer (CheckMate 063): a phase 2, single-arm trial. Lancet Oncol. 2015;16:257–265. doi: 10.1016/S1470-2045(15)70054-9. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

