Pirtobrutinib in relapsed or refractory B-cell malignancies (BRUIN): a phase 1/2 study
- PMID: 33676628
- PMCID: PMC11758240
- DOI: 10.1016/S0140-6736(21)00224-5
Pirtobrutinib in relapsed or refractory B-cell malignancies (BRUIN): a phase 1/2 study
Abstract
Background: Covalent Bruton's tyrosine kinase (BTK) inhibitors are efficacious in multiple B-cell malignancies, but patients discontinue these agents due to resistance and intolerance. We evaluated the safety and efficacy of pirtobrutinib (working name; formerly known as LOXO-305), a highly selective, reversible BTK inhibitor, in these patients.
Methods: Patients with previously treated B-cell malignancies were enrolled in a first-in-human, multicentre, open-label, phase 1/2 trial of the BTK inhibitor pirtobrutinib. The primary endpoint was the maximum tolerated dose (phase 1) and overall response rate (ORR; phase 2). This trial is registered with ClinicalTrials.gov, NCT03740529.
Findings: 323 patients were treated with pirtobrutinib across seven dose levels (25 mg, 50 mg, 100 mg, 150 mg, 200 mg, 250 mg, and 300 mg once per day) with linear dose-proportional exposures. No dose-limiting toxicities were observed and the maximum tolerated dose was not reached. The recommended phase 2 dose was 200 mg daily. Adverse events in at least 10% of 323 patients were fatigue (65 [20%]), diarrhoea (55 [17%]), and contusion (42 [13%]). The most common adverse event of grade 3 or higher was neutropenia (32 [10%]). There was no correlation between pirtobrutinib exposure and the frequency of grade 3 treatment-related adverse events. Grade 3 atrial fibrillation or flutter was not observed, and grade 3 haemorrhage was observed in one patient in the setting of mechanical trauma. Five (1%) patients discontinued treatment due to a treatment-related adverse event. In 121 efficacy evaluable patients with chronic lymphocytic leukaemia (CLL) or small lymphocytic lymphoma (SLL) treated with a previous covalent BTK inhibitor (median previous lines of treatment 4), the ORR with pirtobrutinib was 62% (95% CI 53-71). The ORR was similar in CLL patients with previous covalent BTK inhibitor resistance (53 [67%] of 79), covalent BTK inhibitor intolerance (22 [52%] of 42), BTK C481-mutant (17 [71%] of 24) and BTK wild-type (43 [66%] of 65) disease. In 52 efficacy evaluable patients with mantle cell lymphoma (MCL) previously treated with covalent BTK inhibitors, the ORR was 52% (95% CI 38-66). Of 117 patients with CLL, SLL, or MCL who responded, all but eight remain progression-free to date.
Interpretation: Pirtobrutinib was safe and active in multiple B-cell malignancies, including patients previously treated with covalent BTK inhibitors. Pirtobrutinib might address a growing unmet need for alternative therapies for these patients.
Funding: Loxo Oncology.
Copyright © 2021 Elsevier Ltd. All rights reserved.
Conflict of interest statement
ARM reports grants, personal fees and other from TG Therapeutics, grants and personal fees from Loxo Oncology a wholly owned subsidiary of Eli Lilly and Company, grants and personal fees from Genentech, grants and personal fees from Abbvie, grants and personal fees from AstraZeneca, grants from Sunesis, grants from Regeneron, grants and personal fees from Adaptive, grants and personal fees from Pharmacyclics, non-financial support from NCCN, non-financial support from CLL society, non-financial support from Lymphoma research foundation, grants and personal fees from Curio Sciences, grants from Pfizer, grants and other from Verastem, grants from Aprea, grants from Aptose, grants from DTRM, during the conduct of the study.
NNS reports personal fees from Loxo Oncology a wholly owned subsidiary of Eli Lilly and Company OXO, a wholly owned subsidiary of Eli Lilly and company, during the conduct of the study; personal fees and other from Miltenyi Biotec, personal fees from Incyte, personal fees from Celgene, personal fees from Kite, personal fees from Verastem, outside the submitted work.
WJ reports grants from Janssen, grants from AstraZeneca, grants from Beigene, grants from Loxo Oncology a wholly owned subsidiary of Eli Lilly and Company, during the conduct of the study.
CYC reports personal fees from Loxo Oncology a wholly owned subsidiary of Eli Lilly and Company, during the conduct of the study; grants and personal fees from Roche, grants and personal fees from BMS, personal fees from Takeda, personal fees from AstraZeneca, personal fees from Ascentage Pharma, from TG Therapeutics, grants from Abbvie, personal fees from Janssen, personal fees from Gilead, outside the submitted work.
JMP reports other from Loxo Oncology a wholly owned subsidiary of Eli Lilly and Company, other from AstraZeneca, other from Gilead, other from BeiGene, during the conduct of the study.
JAW reports personal fees from Loxo Oncology a wholly owned subsidiary of Eli Lilly and Company, during the conduct of the study; personal fees from Janssen, Pharmacyclics, AZ, Abbvie, Arqule, grants from Pharmacyclics, Janssen, Morphosys, Karyopharm, Verastem, Abbvie, personal fees from Pharmacyclics, LCC, an Abbvie Company, Abbvie, Janssen, AstraZeneca, ArQule, outside the submitted work.
BF has nothing to disclose.
TAE reports personal fees from Roche, personal fees and other from Gilead, personal fees from Loxo Oncology a wholly owned subsidiary of Eli Lilly and Company, personal fees from Abbvie, personal fees from Janssen, personal fees from Beigene, personal fees from AstraZeneca, personal fees from KITE, outside the submitted work.
NL reports grants from Loxo Oncology a wholly owned subsidiary of Eli Lilly and Company, during the conduct of the study; grants, personal fees and other from Abbvie, AstraZeneca, BeiGene, Genentech, personal fees and other from Celgene, Gilead, Janssen, Pharmacyclics, grants from Juno, Octernal, Verastem, TG Therapeutics, MingSight, grants from Octapharma, outside the submitted work.
MRP reports other from Janssen, other from EMD serono, other from Pfizer, other from Pharmacyclics, other from Bayer, other from Genentech, other from Loxo a wholly owned subsidiary of Eli Lilly and Company, during the conduct of the study. AA reports grants from Loxo Oncology a wholly owned subsidiary of Eli Lilly and Company, during the conduct of the study; personal fees from Karyopharm, personal fees from Kite, other from Kite, personal fees from Amgen, personal fees from Celgene, personal fees from Spectrum Pharmaceuticals, outside the submitted work. ELM reports personal fees from Roche, Novartis, Takeda, Janssen-Cilag, Amgen, Gilead, Abbvie, Sanofi, personal fees from Roche, Amgen, Gilead, outside the submitted work.
WGW has nothing to disclose.
CCC reports personal fees and other from Loxo Oncology a wholly owned subsidiary of Eli Lilly and Company, during the conduct of the study; personal fees from Novartis, personal fees from AbbVie, personal fees from Genentech, personal fees from MEI Pharma, personal fees from Octapharma, other from Gilead, other from H3 Biomedicine, other from Incyte, outside the submitted work.
JNG reports grants from Loxo Oncology a wholly owned subsidiary of Eli Lilly and Company, during the conduct of the study; personal fees from Abbvie, Pharmacyclics, Genentech, outside the submitted work.
PG reports grants and personal fees from AbbVie, personal fees from Adaptive, personal fees from Celgene/Juno, personal fees from AstraZeneca, grants from Gilead, grants and personal fees from Janssen, personal fees from Loxo Oncology a wholly owned subsidiary of Eli Lilly and Company, grants from Novartis, outside the submitted work.
SLG reports personal fees from Loxo Oncology a wholly owned subsidiary of Eli Lilly and Company, during the conduct of the study; personal fees from Roche, Genentech, Janssen-Cilag, Abbvie, Celgene, Jazz, Gilead-KITE, Daiichi-Sankyo, Servier, outside the submitted work.
DJL has nothing to disclose.
SS has nothing to disclose.
JBC reports grants from Loxo Oncology a wholly owned subsidiary of Eli Lilly and Company, during the conduct of the study; grants from Genentech, grants from Astra Zeneca, grants from Novartis, grants from Celgene, grants from M2Gen, grants from BioInvent, grants from Hutchison, personal fees from Adicet, personal fees from Loxo Oncology a wholly owned subsidiary of Eli Lilly and Company, personal fees from Cellectar, personal fees from Kite/Gilead, personal fees from Beigene, personal fees from Aptitude health, personal fees from Pharmacyclics, outside the submitted work. IWF reports grants and other from AbbVie, grants and other from AstraZeneca, grants and other from BeiGene, grants and other from Gilead Sciences, other from Great Point Partners, other from Iksuda Therapeutics, grants and other from Janssen, grants and other from Juno Therapeutics, grants and other from Kite Pharma, grants and other from MorphoSys, other from Nurix Therapeutics, grants and other from Pharmacyclics, grants and other from Roche, grants and other from Seattle Genetics, grants and other from Takeda, grants and other from TG Therapeutics, grants and other from Unum Therapeutics, grants and other from Verastem, other from Yingli Pharmaceuticals, grants from Acerta Pharmaceuticals, grants from Agios, grants from ArQule grants from Calithera Biosciences, grants from Celgene, grants from Constellation Pharmaceuticals, grants from Curis, grants from Forma Therapeutics, grants from Forty Seven, grants from Genentech, grants from IGM Biosciences, grants from Incyte, grants from Infinity Pharmaceuticals, grants from Karyopharm Therapeutics, grants from Loxo Oncology a wholly owned subsidiary of Eli Lilly and Company, grants from Merck, grants from Novartis, grants from Pfizer, grants from Portola Pharmaceuticals, grants from Rhizen Pharmaceuticals, grants from Teva, grants from Trillium Therapeutics, grants from Triphase Research & Development Corp., outside the submitted work.
CST reports grants and personal fees from Janssen, BeiGene, Abbvie, personal fees from Pharmacyclics, outside the submitted work.
MAB has nothing to disclose.
BK reports personal fees from Janssen, personal fees from AstraZeneca, personal fees from AbbVie, personal fees from Kyowa Kirin, personal fees from Mundipharma, outside the submitted work.
JT has nothing to disclose.
OAW reports grants and personal fees from H3 Biomedicine, grants from Loxo Oncology a wholly owned subsidiary of Eli Lilly and Company, personal fees from Pfizer, personal fees from Prelude Therapeutics, personal fees from Foundation Medicine, personal fees from Incyte Pharmaceuticals, personal fees from Envisagenics Inc., personal fees from AI Chemy, during the conduct of the study; grants from LOXO Oncology, outside the submitted work.
SJS reports personal fees from Loxo Oncology a wholly owned subsidiary of Eli Lilly and Company, during the conduct of the study; personal fees from AlloGene, AstraZeneca, BeiGene, Genentech, Inc./ F. Hoffmann-LaRoche, Juno/Celgene, Loxo Oncology a wholly owned subsidiary of Eli Lilly and Company, Nordic Nanovector, Novartis, Tessa Therapeutics, grants from Novartis, Genentech, F Hoffman-La Roche, outside the submitted work.
MLP reports personal fees from Novartis, personal fees and other from Merck & Co. Inc., personal fees and other from Pharmacyclics, personal fees and other from Kite, outside the submitted work; MLP has intellectual property interests (by virtue of immediate family member interests) related to CARs (Chimeric Antigen Receptors) and TCRs (T cell Receptors) that Memorial Sloan Kettering Cancer Center (MSK) has licensed to Juno Therapeutics.
KLL has nothing to disclose.
LER reports minority ownership interest in AbbVie and Abbott Laboratories, personal fees from Vaniam Group, personal fees from Janssen Biotech, grant funding from American Society of Hematology outside of the submitted work.
MSD reports grants and personal fees from AbbVie, personal fees from Adaptive Biotechnologies, grants and personal fees from Ascentage Pharma, grants and personal fees from AstraZeneca, personal fees from BeiGene, personal fees from Celgene, personal fees from Eli Lilly, grants and personal fees from Genentech, personal fees from Gilead Sciences, personal fees from Janssen, grants and personal fees from MEI Pharma, personal fees from Merck, grants and personal fees from Novartis, grants and personal fees from Pharmacyclics, personal fees from Research to Practice, grants from Surface Oncology, personal fees from Syros Pharmaceuticals, grants and personal fees from TG Therapeutics, grants and personal fees from Verastem, personal fees from Zentalis, outside the submitted work.
XNT reports grants from Department of Health, Western Australia, other from Novartis, outside the submitted work.
TSF has nothing to disclose.
DET reports other from Loxo Oncology, a wholly owned subsidiary of Eli Lilly and Company during the conduct of the study.
NCK reports other from Loxo Oncology, a wholly owned subsidiary of Eli Lilly and Company during the conduct of the study.
EZ reports other from Loxo Oncology, a wholly owned subsidiary of Eli Lilly and Company during the conduct of the study.
JC reports other from Loxo Oncology, a wholly owned subsidiary of Eli Lilly and Company during the conduct of the study.
MY reports other from Loxo Oncology, a wholly owned subsidiary of Eli Lilly and Company during the conduct of the study.
BN reports other from Loxo Oncology, a wholly owned subsidiary of Eli Lilly and Company during the conduct of the study.
KE reports other from Loxo Oncology, a wholly owned subsidiary of Eli Lilly and Company during the conduct of the study.
NM reports other from Loxo Oncology, a wholly owned subsidiary of Eli Lilly and Company during the conduct of the study.
JRB reports personal fees from Abbvie, personal fees from Acerta/AstraZeneca, personal fees from Astellas, personal fees from BeiGene, personal fees from Catapult Therapeutics, personal fees from Dynamo Therapeutics, personal fees from Genentech/Roche, grants and personal fees from Gilead, other from Invectys, personal fees from Janssen, personal fees from Juno/Celgene, personal fees from Kite, other from Morphosys, personal fees from Novartis, personal fees from Octapharma, personal fees from Pfizer, personal fees from Pharmacyclics, personal fees from Redx, grants from Sun, personal fees from Sunesis, personal fees from Teva, personal fees from TG Therapeutics, grants and personal fees from Verastem, personal fees from MEI Pharma, personal fees from Nextcea, personal fees from Rigel, grants and personal fees from Loxo Oncology a wholly owned subsidiary of Eli Lilly and Company, outside the submitted work.
MW reports grants, personal fees and non-financial support from Pharmacyclics, grants, personal fees and non-financial support from Celgene, grants, personal fees and non-financial support from Janssen, grants and personal fees from AstraZeneca/Acerta Pharma, personal fees and non-financial support from OMI, personal fees from Pulse Biosciences, grants and personal fees from Juno, grants and personal fees from Loxo Oncology a wholly owned subsidiary of Eli Lilly and Company, grants and personal fees from VelosBio, grants from BioInvent, personal fees from Targeted Oncology, grants and personal fees from Kite Pharma, personal fees from Guidepoint Global, grants from Verastem, grants and personal fees from BeiGene, grants from Eli Lilly, grants and personal fees from InnoCare, grants from Molecular Templates, grants and personal fees from Oncternal, outside the submitted work.
Figures
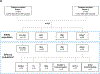

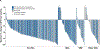

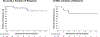
Comment in
-
Pirtobrutinib shows evidence to inaugurate a third generation of BTK inhibitors.Lancet. 2021 Mar 6;397(10277):855-857. doi: 10.1016/S0140-6736(21)00235-X. Lancet. 2021. PMID: 33676615 No abstract available.
-
Initial pirtobrutinib data show promise.Nat Rev Clin Oncol. 2021 May;18(5):258. doi: 10.1038/s41571-021-00505-0. Nat Rev Clin Oncol. 2021. PMID: 33758377 No abstract available.
Similar articles
-
Pirtobrutinib, a highly selective, non-covalent (reversible) BTK inhibitor in patients with B-cell malignancies: analysis of the Richter transformation subgroup from the multicentre, open-label, phase 1/2 BRUIN study.Lancet Haematol. 2024 Sep;11(9):e682-e692. doi: 10.1016/S2352-3026(24)00172-8. Epub 2024 Jul 18. Lancet Haematol. 2024. PMID: 39033770 Clinical Trial.
-
Pirtobrutinib after a Covalent BTK Inhibitor in Chronic Lymphocytic Leukemia.N Engl J Med. 2023 Jul 6;389(1):33-44. doi: 10.1056/NEJMoa2300696. N Engl J Med. 2023. PMID: 37407001 Clinical Trial.
-
Pirtobrutinib monotherapy in Bruton tyrosine kinase inhibitor-intolerant patients with B-cell malignancies: results of the phase I/II BRUIN trial.Haematologica. 2025 Jan 1;110(1):92-102. doi: 10.3324/haematol.2024.285754. Haematologica. 2025. PMID: 39363864 Free PMC article. Clinical Trial.
-
Oral budesonide for induction of remission in ulcerative colitis.Cochrane Database Syst Rev. 2015 Oct 26;2015(10):CD007698. doi: 10.1002/14651858.CD007698.pub3. Cochrane Database Syst Rev. 2015. PMID: 26497719 Free PMC article. Review.
-
Maintenance therapy for chronic lymphocytic leukaemia.Cochrane Database Syst Rev. 2024 Jan 4;1(1):CD013474. doi: 10.1002/14651858.CD013474.pub2. Cochrane Database Syst Rev. 2024. PMID: 38174814 Free PMC article. Review.
Cited by
-
Resistance to Bruton tyrosine kinase inhibition in chronic lymphocytic leukaemia and non-Hodgkin lymphoma.Br J Haematol. 2023 Jan;200(2):137-149. doi: 10.1111/bjh.18418. Epub 2022 Aug 27. Br J Haematol. 2023. PMID: 36029036 Free PMC article. Review.
-
The role of allogeneic hematopoietic cell transplantation for chronic lymphocytic leukemia: A review.Front Oncol. 2023 Jan 18;12:1105779. doi: 10.3389/fonc.2022.1105779. eCollection 2022. Front Oncol. 2023. PMID: 36741737 Free PMC article. Review.
-
High rate of durable responses with undetectable minimal residual disease with front-line venetoclax and rituximab in young, fit patients with chronic lymphocytic leukemia and an adverse biological profile: results of the GIMEMA phase II LLC1518 - VERITAS study.Haematologica. 2023 Aug 1;108(8):2091-2100. doi: 10.3324/haematol.2022.282116. Haematologica. 2023. PMID: 36632738 Free PMC article. Clinical Trial.
-
A year in pharmacology: new drugs approved by the US Food and Drug Administration in 2023.Naunyn Schmiedebergs Arch Pharmacol. 2024 May;397(5):2949-2970. doi: 10.1007/s00210-024-03063-1. Epub 2024 Mar 26. Naunyn Schmiedebergs Arch Pharmacol. 2024. PMID: 38530400 Free PMC article. Review.
-
Pirtobrutinib: First Approval.Drugs. 2023 Apr;83(6):547-553. doi: 10.1007/s40265-023-01860-1. Drugs. 2023. PMID: 37004673 Review.
References
-
- Treon SP, Tripsas CK, Meid K, et al. Ibrutinib in previously treated Waldenstrom’s macroglobulinemia. N Engl J Med 2015;372:1430–40. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

