C9orf72 ALS-FTD: recent evidence for dysregulation of the autophagy-lysosome pathway at multiple levels
- PMID: 33632058
- PMCID: PMC8632097
- DOI: 10.1080/15548627.2021.1872189
C9orf72 ALS-FTD: recent evidence for dysregulation of the autophagy-lysosome pathway at multiple levels
Abstract
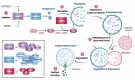
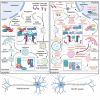
Amyotrophic lateral sclerosis (ALS) and frontotemporal dementia (FTD) are two clinically distinct classes of neurodegenerative disorders. Yet, they share a range of genetic, cellular, and molecular features. Hexanucleotide repeat expansions (HREs) in the C9orf72 gene and the accumulation of toxic protein aggregates in the nervous systems of the affected individuals are among such common features. Though the mechanisms by which HREs cause toxicity is not clear, the toxic gain of function due to transcribed HRE RNA or dipeptide repeat proteins (DPRs) produced by repeat-associated non-AUG translation together with a reduction in C9orf72 expression are proposed as the contributing factors for disease pathogenesis in ALS and FTD. In addition, several recent studies point toward alterations in protein homeostasis as one of the root causes of the disease pathogenesis. In this review, we discuss the effects of the C9orf72 HRE in the autophagy-lysosome pathway based on various recent findings. We suggest that dysfunction of the autophagy-lysosome pathway synergizes with toxicity from C9orf72 repeat RNA and DPRs to drive disease pathogenesis.Abbreviation: ALP: autophagy-lysosome pathway; ALS: amyotrophic lateral sclerosis; AMPK: AMP-activated protein kinase; ATG: autophagy-related; ASO: antisense oligonucleotide; C9orf72: C9orf72-SMCR8 complex subunit; DENN: differentially expressed in normal and neoplastic cells; DPR: dipeptide repeat protein; EIF2A/eIF2α: eukaryotic translation initiation factor 2A; ER: endoplasmic reticulum; FTD: frontotemporal dementia; GAP: GTPase-activating protein; GEF: guanine nucleotide exchange factor; HRE: hexanucleotide repeat expansion; iPSC: induced pluripotent stem cell; ISR: integrated stress response; M6PR: mannose-6-phosphate receptor, cation dependent; MAP1LC3/LC3: microtubule associated protein 1 light chain 3; MN: motor neuron; MTORC1: mechanistic target of rapamycin kinase complex 1; ND: neurodegenerative disorder; RAN: repeat-associated non-ATG; RB1CC1/FIP200: RB1 inducible coiled-coil 1; SLC66A1/PQLC2: solute carrier family 66 member 1; SMCR8: SMCR8-C9orf72 complex subunit; SQSTM1/p62: sequestosome 1; STX17: syntaxin 17; TARDBP/TDP-43: TAR DNA binding protein; TBK1: TANK binding kinase 1; TFEB: transcription factor EB; ULK1: unc-51 like autophagy activating kinase 1; UPS: ubiquitin-proteasome system; WDR41: WD repeat domain 41.
Keywords: Amyotrophic lateral sclerosis (ALS); autophagy; axonal transport; c9orf72; dipeptide repeat protein (DPR); frontotemporal dementia (FTD); lysosome; smcr8; wdr41.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
C9orf72 and smcr8 mutant mice reveal MTORC1 activation due to impaired lysosomal degradation and exocytosis.Autophagy. 2020 Sep;16(9):1635-1650. doi: 10.1080/15548627.2019.1703353. Epub 2019 Dec 26. Autophagy. 2020. PMID: 31847700 Free PMC article.
-
The most prevalent genetic cause of ALS-FTD, C9orf72 synergizes the toxicity of ATXN2 intermediate polyglutamine repeats through the autophagy pathway.Autophagy. 2016 Aug 2;12(8):1406-8. doi: 10.1080/15548627.2016.1189070. Epub 2016 May 31. Autophagy. 2016. PMID: 27245636 Free PMC article.
-
Toxic gain-of-function mechanisms in C9orf72 ALS-FTD neurons drive autophagy and lysosome dysfunction.Autophagy. 2024 Sep;20(9):2102-2104. doi: 10.1080/15548627.2024.2340415. Epub 2024 Apr 18. Autophagy. 2024. PMID: 38615685 Free PMC article.
-
The progress in C9orf72 research: ALS/FTD pathogenesis, functions and structure.Small GTPases. 2022 Jan;13(1):56-76. doi: 10.1080/21541248.2021.1892443. Epub 2021 Mar 5. Small GTPases. 2022. PMID: 33663328 Free PMC article. Review.
-
Lysosome dysfunction as a cause of neurodegenerative diseases: Lessons from frontotemporal dementia and amyotrophic lateral sclerosis.Neurobiol Dis. 2021 Jul;154:105360. doi: 10.1016/j.nbd.2021.105360. Epub 2021 Mar 31. Neurobiol Dis. 2021. PMID: 33812000 Free PMC article. Review.
Cited by
-
The Advance on Frontotemporal Dementia (FTD)'s Neuropathology and Molecular Genetics.Mediators Inflamm. 2022 Oct 13;2022:5003902. doi: 10.1155/2022/5003902. eCollection 2022. Mediators Inflamm. 2022. PMID: 36274975 Free PMC article. Review.
-
Pectolinarigenin Improves Oxidative Stress and Apoptosis in Mouse NSC-34 Motor Neuron Cell Lines Induced by C9-ALS-Associated Proline-Arginine Dipeptide Repeat Proteins by Enhancing Mitochondrial Fusion Mediated via the SIRT3/OPA1 Axis.Antioxidants (Basel). 2023 Nov 16;12(11):2008. doi: 10.3390/antiox12112008. Antioxidants (Basel). 2023. PMID: 38001861 Free PMC article.
-
CYP2E1 deficit mediates cholic acid-induced malignant growth in hepatocellular carcinoma cells.Mol Med. 2024 Jun 7;30(1):79. doi: 10.1186/s10020-024-00844-5. Mol Med. 2024. PMID: 38844847 Free PMC article.
-
Staufen Impairs Autophagy in Neurodegeneration.Ann Neurol. 2023 Feb;93(2):398-416. doi: 10.1002/ana.26515. Epub 2022 Oct 27. Ann Neurol. 2023. PMID: 36151701 Free PMC article.
-
C9orf72 Hexanucleotide Repeat Expansion-Related Neuropathology Is Attenuated by Nasal Rifampicin in Mice.Biomedicines. 2022 May 6;10(5):1080. doi: 10.3390/biomedicines10051080. Biomedicines. 2022. PMID: 35625816 Free PMC article.
References
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
