Molecular Definition of Pseudohypoparathyroidism Variants
- PMID: 33529330
- PMCID: PMC8118362
- DOI: 10.1210/clinem/dgab060
Molecular Definition of Pseudohypoparathyroidism Variants
Abstract
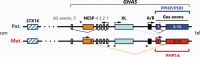
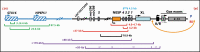
Pseudohypoparathyroidism (PHP) and pseudopseudohypoparathyroidism (PPHP) are caused by mutations and/or epigenetic changes at the complex GNAS locus on chromosome 20q13.3 that undergoes parent-specific methylation changes at several differentially methylated regions (DMRs). GNAS encodes the alpha-subunit of the stimulatory G protein (Gsα) and several splice variants thereof. PHP type Ia (PHP1A) is caused by heterozygous inactivating mutations involving the maternal exons 1-13. Heterozygosity of these maternal GNAS mutations cause PTH-resistant hypocalcemia and hyperphosphatemia because paternal Gsα expression is suppressed in certain organs thus leading to little or no Gsα protein in the proximal renal tubules and other tissues. Besides biochemical abnormalities, PHP1A patients show developmental abnormalities, referred to as Albright's hereditary osteodystrophy (AHO). Some, but not all of these AHO features are encountered also in patients affected by PPHP, who carry paternal Gsα-specific mutations and typically show no laboratory abnormalities. Autosomal dominant PHP type Ib (AD-PHP1B) is caused by heterozygous maternal deletions within GNAS or STX16, which are associated with loss of methylation at the A/B DMR alone or at all maternally methylated GNAS exons. Loss of methylation of exon A/B and the resulting biallelic expression of A/B transcript reduces Gsα expression thus leading to hormonal resistance. Epigenetic changes at all differentially methylated GNAS regions are also observed in sporadic PHP1B, which is the most frequent PHP1B variant. However, this disease variant remains unresolved at the molecular level, except for rare cases with paternal uniparental isodisomy or heterodisomy of chromosome 20q (patUPD20q).
Keywords: GNAS; STX16; Gs-alpha; PTH; TSH; cAMP; calcium; epigenetics; parent-specific GNAS methylation; phosphate; pseudohypoparathyroidism.
© The Author(s) 2021. Published by Oxford University Press on behalf of the Endocrine Society.
Figures
Similar articles
-
Pseudohypoparathyroidism: one gene, several syndromes.J Endocrinol Invest. 2017 Apr;40(4):347-356. doi: 10.1007/s40618-016-0588-4. Epub 2016 Dec 19. J Endocrinol Invest. 2017. PMID: 27995443 Review.
-
Pseudohypoparathyroidism: complex disease variants with unfortunate names.J Mol Endocrinol. 2023 Dec 12;72(1):e230104. doi: 10.1530/JME-23-0104. Print 2024 Jan 1. J Mol Endocrinol. 2023. PMID: 37965945 Free PMC article. Review.
-
A Large Inversion Involving GNAS Exon A/B and All Exons Encoding Gsα Is Associated With Autosomal Dominant Pseudohypoparathyroidism Type Ib (PHP1B).J Bone Miner Res. 2017 Apr;32(4):776-783. doi: 10.1002/jbmr.3083. Epub 2017 Feb 24. J Bone Miner Res. 2017. PMID: 28084650 Free PMC article.
-
The GNAS locus and pseudohypoparathyroidism.Adv Exp Med Biol. 2008;626:27-40. doi: 10.1007/978-0-387-77576-0_3. Adv Exp Med Biol. 2008. PMID: 18372789 Review.
-
Similar frequency of paternal uniparental disomy involving chromosome 20q (patUPD20q) in Japanese and Caucasian patients affected by sporadic pseudohypoparathyroidism type Ib (sporPHP1B).Bone. 2015 Oct;79:15-20. doi: 10.1016/j.bone.2015.05.011. Epub 2015 May 19. Bone. 2015. PMID: 25997889 Free PMC article.
Cited by
-
New insights into thyroid dysfunction in patients with inactivating parathyroid hormone/parathyroid hormone-related protein signalling disorder (the hormonal and ultrasound aspects): One-centre preliminary results.Front Endocrinol (Lausanne). 2022 Sep 23;13:1012658. doi: 10.3389/fendo.2022.1012658. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 36213284 Free PMC article.
-
Structural and Functional Implication of Natural Variants of Gαs.Int J Mol Sci. 2023 Feb 17;24(4):4064. doi: 10.3390/ijms24044064. Int J Mol Sci. 2023. PMID: 36835474 Free PMC article.
-
The Diagnosis of Albright's Osteodystrophy in a Case With Respiratory Failure.Cureus. 2024 Feb 29;16(2):e55200. doi: 10.7759/cureus.55200. eCollection 2024 Feb. Cureus. 2024. PMID: 38558694 Free PMC article.
-
Targeted Long-Read Sequencing Identifies a Retrotransposon Insertion as a Cause of Altered GNAS Exon A/B Methylation in a Family With Autosomal Dominant Pseudohypoparathyroidism Type 1b (PHP1B).J Bone Miner Res. 2022 Sep;37(9):1711-1719. doi: 10.1002/jbmr.4647. Epub 2022 Aug 3. J Bone Miner Res. 2022. PMID: 35811283 Free PMC article.
-
Obesity and Gαs Variants.N Engl J Med. 2021 Oct 21;385(17):1619-1622. doi: 10.1056/NEJMe2113123. Epub 2021 Oct 6. N Engl J Med. 2021. PMID: 34614325 Free PMC article. No abstract available.
References
-
- Weinstein LS, Liu J, Sakamoto A, Xie T, Chen M. Minireview: GNAS: normal and abnormal functions. Endocrinology. 2004;145(12):5459-5464. - PubMed
-
- Levine M. Hypoparathyroidism and pseudohypoparathyroidism. In: DeGroot LJ, Jameson LJ. ed. Endocrinology. 5th ed. W.B. Saunders Co; 2005.
-
- Bastepe M, Jüppner H. Pseudohypoparathyroidism, Albright’s hereditary osteodystrophy, and progressive osseous heteroplasia: disorders caused by inactivating GNAS mutations. In: DeGroot LJ, Jameson JL, eds. Endocrinology. Vol. 1, 7th ed. W.B. Saunders Company; 2016:1147-1159.
-
- Shore E, Ahn J, Jan de Beur S, et al. . Paternally-inherited inactivating mutations of the GNAS1 gene in progressive osseous heteroplasia. New Engl J Med. 2002;346(2):99-106. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

