Efficacy of Margetuximab vs Trastuzumab in Patients With Pretreated ERBB2-Positive Advanced Breast Cancer: A Phase 3 Randomized Clinical Trial
- PMID: 33480963
- PMCID: PMC7823434
- DOI: 10.1001/jamaoncol.2020.7932
Efficacy of Margetuximab vs Trastuzumab in Patients With Pretreated ERBB2-Positive Advanced Breast Cancer: A Phase 3 Randomized Clinical Trial
Abstract
Importance: ERRB2 (formerly HER2)-positive advanced breast cancer (ABC) remains typically incurable with optimal treatment undefined in later lines of therapy. The chimeric antibody margetuximab shares ERBB2 specificity with trastuzumab but incorporates an engineered Fc region to increase immune activation.
Objective: To compare the clinical efficacy of margetuximab vs trastuzumab, each with chemotherapy, in patients with pretreated ERBB2-positive ABC.
Design, setting, and participants: The SOPHIA phase 3 randomized open-label trial of margetuximab plus chemotherapy vs trastuzumab plus chemotherapy enrolled 536 patients from August 26, 2015, to October 10, 2018, at 166 sites in 17 countries. Eligible patients had disease progression on 2 or more prior anti-ERBB2 therapies and 1 to 3 lines of therapy for metastatic disease. Data were analyzed from February 2019 to October 2019.
Interventions: Investigators selected chemotherapy before 1:1 randomization to margetuximab, 15 mg/kg, or trastuzumab, 6 mg/kg (loading dose, 8 mg/kg), each in 3-week cycles. Stratification factors were metastatic sites (≤2, >2), lines of therapy (≤2, >2), and chemotherapy choice.
Main outcomes and measures: Sequential primary end points were progression-free survival (PFS) by central blinded analysis and overall survival (OS). All α was allocated to PFS, followed by OS. Secondary end points were investigator-assessed PFS and objective response rate by central blinded analysis.
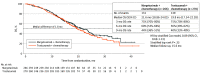
Results: A total of 536 patients were randomized to receive margetuximab (n = 266) or trastuzumab (n = 270). The median age was 56 (27-86) years; 266 (100%) women were in the margetuximab group, while 267 (98.9%) women were in the trastuzumab group. Groups were balanced. All but 1 patient had received prior pertuzumab, and 489 (91.2%) had received prior ado-trastuzumab emtansine. Margetuximab improved primary PFS over trastuzumab with 24% relative risk reduction (hazard ratio [HR], 0.76; 95% CI, 0.59-0.98; P = .03; median, 5.8 [95% CI, 5.5-7.0] months vs 4.9 [95% CI, 4.2-5.6] months; October 10, 2018). After the second planned interim analysis of 270 deaths, median OS was 21.6 months with margetuximab vs 19.8 months with trastuzumab (HR, 0.89; 95% CI, 0.69-1.13; P = .33; September 10, 2019), and investigator-assessed PFS showed 29% relative risk reduction favoring margetuximab (HR, 0.71; 95% CI, 0.58-0.86; P < .001; median, 5.7 vs 4.4 months; September 10, 2019). Margetuximab improved objective response rate over trastuzumab: 22% vs 16% (P = .06; October 10, 2018), and 25% vs 14% (P < .001; September 10, 2019). Incidence of infusion-related reactions, mostly in cycle 1, was higher with margetuximab (35 [13.3%] vs 9 [3.4%]); otherwise, safety was comparable.
Conclusions and relevance: In this phase 3 randomized clinical trial, margetuximab plus chemotherapy had acceptable safety and a statistically significant improvement in PFS compared with trastuzumab plus chemotherapy in ERBB2-positive ABC after progression on 2 or more prior anti-ERBB2 therapies. Final OS analysis is expected in 2021.
Trial registration: ClinicalTrials.gov Identifier: NCT02492711.
Conflict of interest statement
Figures

Similar articles
-
Margetuximab Versus Trastuzumab in Patients With Previously Treated HER2-Positive Advanced Breast Cancer (SOPHIA): Final Overall Survival Results From a Randomized Phase 3 Trial.J Clin Oncol. 2023 Jan 10;41(2):198-205. doi: 10.1200/JCO.21.02937. Epub 2022 Nov 4. J Clin Oncol. 2023. PMID: 36332179 Free PMC article. Clinical Trial.
-
Pertuzumab Plus Trastuzumab With or Without Chemotherapy Followed by Emtansine in ERBB2-Positive Metastatic Breast Cancer: A Secondary Analysis of a Randomized Clinical Trial.JAMA Oncol. 2023 Oct 1;9(10):1381-1389. doi: 10.1001/jamaoncol.2023.2909. JAMA Oncol. 2023. PMID: 37561451 Free PMC article. Clinical Trial.
-
Efficacy and Safety of Gemcitabine With Trastuzumab and Pertuzumab After Prior Pertuzumab-Based Therapy Among Patients With Human Epidermal Growth Factor Receptor 2-Positive Metastatic Breast Cancer: A Phase 2 Clinical Trial.JAMA Netw Open. 2019 Nov 1;2(11):e1916211. doi: 10.1001/jamanetworkopen.2019.16211. JAMA Netw Open. 2019. PMID: 31774522 Free PMC article. Clinical Trial.
-
Margetuximab for the treatment of HER2-positive metastatic breast cancer.Expert Opin Biol Ther. 2021 Feb;21(2):127-133. doi: 10.1080/14712598.2021.1856812. Epub 2020 Dec 14. Expert Opin Biol Ther. 2021. PMID: 33238772 Review.
-
Metastatic Human Epidermal Growth Factor Receptor 2-Positive Breast Cancer: Current Treatment Standards and Future Perspectives.Breast Care (Basel). 2020 Dec;15(6):570-578. doi: 10.1159/000512328. Epub 2020 Nov 12. Breast Care (Basel). 2020. PMID: 33447230 Free PMC article. Review.
Cited by
-
Breast cancer immunotherapy: Realities and advances.Cancer Innov. 2024 Sep 22;3(5):e140. doi: 10.1002/cai2.140. eCollection 2024 Oct. Cancer Innov. 2024. PMID: 39308754 Free PMC article. Review.
-
Margetuximab Versus Trastuzumab in Patients With Previously Treated HER2-Positive Advanced Breast Cancer (SOPHIA): Final Overall Survival Results From a Randomized Phase 3 Trial.J Clin Oncol. 2023 Jan 10;41(2):198-205. doi: 10.1200/JCO.21.02937. Epub 2022 Nov 4. J Clin Oncol. 2023. PMID: 36332179 Free PMC article. Clinical Trial.
-
Therapeutic Landscape of Human Epidermal Growth Factor Receptor 2-Positive Breast Cancer.Cancer Control. 2022 Jan-Dec;29:10732748221099230. doi: 10.1177/10732748221099230. Cancer Control. 2022. PMID: 35499382 Free PMC article.
-
New and Emerging Targeted Therapies for Advanced Breast Cancer.Int J Mol Sci. 2022 Feb 18;23(4):2288. doi: 10.3390/ijms23042288. Int J Mol Sci. 2022. PMID: 35216405 Free PMC article. Review.
-
Targeting mutations in cancer.J Clin Invest. 2022 Apr 15;132(8):e154943. doi: 10.1172/JCI154943. J Clin Invest. 2022. PMID: 35426374 Free PMC article. Review.
References
-
- National Comprehensive Cancer Network . NCCN Clinical Practice Guidelines in Oncology: breast cancer (v3.2020). Accessed March 13, 2020. https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

