Development of a Bioprocess for the Production of Cyclic Lipopeptides Pseudofactins With Efficient Purification From Collected Foam
- PMID: 33330412
- PMCID: PMC7719756
- DOI: 10.3389/fbioe.2020.565619
Development of a Bioprocess for the Production of Cyclic Lipopeptides Pseudofactins With Efficient Purification From Collected Foam
Abstract
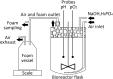
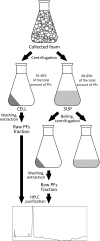
Microbial surfactants (biosurfactants) have gained interest as promising substitutes of synthetic surface-active compounds. However, their production and purification are still challenging, with significant room for efficiency and costs optimization. In this work, we introduce a method for the enhanced production and purification of cyclic lipopeptides pseudofactins (PFs) from Pseudomonas fluorescens BD5 cultures. The method is directly applicable in a technical scale with the possibility of further upscaling. Comparing to the original protocol for production of PFs (cultures in mineral salt medium in shaken flasks followed by solvent-solvent extraction of PFs), our process offers not only ∼24-fold increased productivity, but also easier and more efficient purification. The new process combines high yield of PFs (∼7.2 grams of PFs per 30 L of working volume), with recovery levels of 80-90% and purity of raw PFs up to 60-70%. These were achieved with an innovative, single-step thermal co-precipitation and extraction of PFs directly from collected foam, as a large amount of PF-enriched foam was produced during the bioprocess. Besides we present a protocol for the selective production of PF structural analogs and their separation with high-performance liquid chromatography. Our approach can be potentially utilized in the efficient production and purification of other lipopeptides of Pseudomonas and Bacillus origin.
Keywords: Pseudomonas fluorescens; bioreactor; biosurfactant production; cyclic lipopeptides (CLPs); foam fractionation; lipopeptide production; lipopeptide purification.
Copyright © 2020 Biniarz, Henkel, Hausmann and Łukaszewicz.
Figures


Similar articles
-
High-throughput optimization of medium components and culture conditions for the efficient production of a lipopeptide pseudofactin by Pseudomonas fluorescens BD5.Microb Cell Fact. 2018 Aug 4;17(1):121. doi: 10.1186/s12934-018-0968-x. Microb Cell Fact. 2018. PMID: 30077177 Free PMC article.
-
Isolation and characterization of two new lipopeptide biosurfactants produced by Pseudomonas fluorescens BD5 isolated from water from the Arctic Archipelago of Svalbard.Bioresour Technol. 2010 Aug;101(15):6118-23. doi: 10.1016/j.biortech.2010.02.109. Epub 2010 Mar 19. Bioresour Technol. 2010. PMID: 20303744
-
Production of lipopeptide biosurfactants by Bacillus atrophaeus 5-2a and their potential use in microbial enhanced oil recovery.Microb Cell Fact. 2016 Oct 3;15(1):168. doi: 10.1186/s12934-016-0574-8. Microb Cell Fact. 2016. PMID: 27716284 Free PMC article.
-
Process development and intensification for enhanced production of Bacillus lipopeptides.Biotechnol Genet Eng Rev. 2015 Apr-Oct;31(1-2):46-68. doi: 10.1080/02648725.2016.1166335. Epub 2016 May 3. Biotechnol Genet Eng Rev. 2015. PMID: 27136722 Review.
-
In situ downstream strategies for cost-effective bio/surfactant recovery.Biotechnol Appl Biochem. 2018 Jul;65(4):523-532. doi: 10.1002/bab.1641. Epub 2018 Feb 21. Biotechnol Appl Biochem. 2018. PMID: 29297935 Review.
Cited by
-
Uncoupling Foam Fractionation and Foam Adsorption for Enhanced Biosurfactant Synthesis and Recovery.Microorganisms. 2020 Dec 18;8(12):2029. doi: 10.3390/microorganisms8122029. Microorganisms. 2020. PMID: 33353027 Free PMC article.
-
Does regulation hold the key to optimizing lipopeptide production in Pseudomonas for biotechnology?Front Bioeng Biotechnol. 2024 Feb 27;12:1363183. doi: 10.3389/fbioe.2024.1363183. eCollection 2024. Front Bioeng Biotechnol. 2024. PMID: 38476965 Free PMC article. Review.
-
New insight into the role of oxygen supply for surfactin production in bench-scale bioreactors using induced surface aeration.Bioprocess Biosyst Eng. 2022 Dec;45(12):2031-2041. doi: 10.1007/s00449-022-02807-8. Epub 2022 Nov 7. Bioprocess Biosyst Eng. 2022. PMID: 36342535
-
New Continuous Process for the Production of Lipopeptide Biosurfactants in Foam Overflowing Bioreactor.Front Bioeng Biotechnol. 2021 May 28;9:678469. doi: 10.3389/fbioe.2021.678469. eCollection 2021. Front Bioeng Biotechnol. 2021. PMID: 34124025 Free PMC article.
-
Scalable downstream method for the cyclic lipopetide jagaricin.Eng Life Sci. 2021 Oct 27;22(12):811-817. doi: 10.1002/elsc.202100079. eCollection 2022 Dec. Eng Life Sci. 2021. PMID: 36514532 Free PMC article.
References
-
- Alonso S., Martin P. J. (2016). Impact of foaming on surfactin production by bacillus subtilis: implications on the development of integrated in situ foam fractionation removal systems. Biochem. Eng. J. 110 125–133. 10.1016/j.bej.2016.02.006 - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

