Xylose-Inducible Promoter Tools for Pseudomonas Species and Their Use in Implicating a Role for the Type II Secretion System Protein XcpQ in the Inhibition of Corneal Epithelial Wound Closure
- PMID: 32414795
- PMCID: PMC7357479
- DOI: 10.1128/AEM.00250-20
Xylose-Inducible Promoter Tools for Pseudomonas Species and Their Use in Implicating a Role for the Type II Secretion System Protein XcpQ in the Inhibition of Corneal Epithelial Wound Closure
Abstract
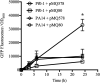
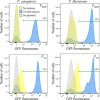
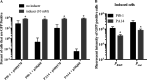
Tunable control of gene expression is an invaluable tool for biological experiments. In this study, we describe a new xylose-inducible promoter system and evaluate it in both Pseudomonas aeruginosa and Pseudomonas fluorescens The Pxut promoter, derived from the P. fluorescensxut operon, was incorporated into a broad-host-range pBBR1-based plasmid and was compared to the Escherichia coli-derived PBAD promoter using gfp as a reporter. Green fluorescent protein (GFP) fluorescence from the Pxut promoter was inducible in both Pseudomonas species, but not in E. coli, which may facilitate the cloning of genes toxic to E. coli to generate plasmids. The Pxut promoter was activated at a lower inducer concentration than PBAD in P. fluorescens, and higher gfp levels were achieved using Pxut Flow cytometry analysis indicated that Pxut was leakier than PBAD in the Pseudomonas species tested but was expressed in a higher proportion of cells when induced. d-Xylose as a sole carbon source did not support the growth of P. aeruginosa or P. fluorescens and is less expensive than many other commonly used inducers, which could facilitate large-scale applications. The efficacy of this system was demonstrated by its use to reveal a role for the P. aeruginosa type II secretion system gene xcpQ in bacterial inhibition of corneal epithelial cell wound closure. This study introduces a new inducible promoter system for gene expression for use in Pseudomonas species.IMPORTANCEPseudomonas species are enormously important in human infections, in biotechnology, and as model systems for investigating basic science questions. In this study, we have developed a xylose-inducible promoter system, evaluated it in P. aeruginosa and P. fluorescens, and found it to be suitable for the strong induction of gene expression. Furthermore, we have demonstrated its efficacy in controlled gene expression to show that a type II secretion system protein from P. aeruginosa, XcpQ, is important for host-pathogen interactions in a corneal wound closure model.
Keywords: Pseudomonas; inducible promoter; plasmids; xylose.
Copyright © 2020 American Society for Microbiology.
Figures



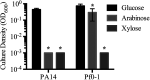

Similar articles
-
Influence of a putative ECF sigma factor on expression of the major outer membrane protein, OprF, in Pseudomonas aeruginosa and Pseudomonas fluorescens.J Bacteriol. 1999 Aug;181(16):4746-54. doi: 10.1128/JB.181.16.4746-4754.1999. J Bacteriol. 1999. PMID: 10438740 Free PMC article.
-
Unraveling the Self-Assembly of the Pseudomonas aeruginosa XcpQ Secretin Periplasmic Domain Provides New Molecular Insights into Type II Secretion System Secreton Architecture and Dynamics.mBio. 2017 Oct 17;8(5):e01185-17. doi: 10.1128/mBio.01185-17. mBio. 2017. PMID: 29042493 Free PMC article.
-
The Escherichia coli rhaSR-PrhaBAD Inducible Promoter System Allows Tightly Controlled Gene Expression over a Wide Range in Pseudomonas aeruginosa.Appl Environ Microbiol. 2016 Oct 27;82(22):6715-6727. doi: 10.1128/AEM.02041-16. Print 2016 Nov 15. Appl Environ Microbiol. 2016. PMID: 27613678 Free PMC article.
-
Protein secretion systems of Pseudomonas aeruginosa and P fluorescens.Biochim Biophys Acta. 2003 Apr 1;1611(1-2):223-33. doi: 10.1016/s0005-2736(03)00059-2. Biochim Biophys Acta. 2003. PMID: 12659964 Review.
-
Regulation of biofilm formation in Pseudomonas and Burkholderia species.Environ Microbiol. 2014 Jul;16(7):1961-81. doi: 10.1111/1462-2920.12448. Epub 2014 Mar 31. Environ Microbiol. 2014. PMID: 24592823 Review.
Cited by
-
Antibiotics Used in Empiric Treatment of Ocular Infections Trigger the Bacterial Rcs Stress Response System Independent of Antibiotic Susceptibility.Antibiotics (Basel). 2021 Aug 25;10(9):1033. doi: 10.3390/antibiotics10091033. Antibiotics (Basel). 2021. PMID: 34572615 Free PMC article.
-
The type II secretion system as an underappreciated and understudied mediator of interbacterial antagonism.Infect Immun. 2024 Aug 13;92(8):e0020724. doi: 10.1128/iai.00207-24. Epub 2024 Jul 9. Infect Immun. 2024. PMID: 38980047 Free PMC article. Review.
-
Suppressor mutants demonstrate the metabolic plasticity of unsaturated fatty acid synthesis in Pseudomonas aeruginosa PAO1.Microbiology (Reading). 2023 Oct;169(10):001400. doi: 10.1099/mic.0.001400. Microbiology (Reading). 2023. PMID: 37818937 Free PMC article.
-
Development of a xylose-inducible promoter and riboswitch combination system for manipulating gene expression in Fusobacterium nucleatum.Appl Environ Microbiol. 2023 Sep 28;89(9):e0066723. doi: 10.1128/aem.00667-23. Epub 2023 Sep 11. Appl Environ Microbiol. 2023. PMID: 37695289 Free PMC article.
-
Development of a luciferase-based Gram-positive bacterial reporter system for the characterization of antimicrobial agents.Appl Environ Microbiol. 2024 Aug 21;90(8):e0071724. doi: 10.1128/aem.00717-24. Epub 2024 Jul 17. Appl Environ Microbiol. 2024. PMID: 39016615 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

