RETRACTED: Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial
- PMID: 32205204
- PMCID: PMC7102549
- DOI: 10.1016/j.ijantimicag.2020.105949
RETRACTED: Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial
Retraction in
-
Retraction notice to "Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial" [International Journal of Antimicrobial Agents 56 (2020), 105949].Int J Antimicrob Agents. 2025 Jan;65(1):107416. doi: 10.1016/j.ijantimicag.2024.107416. Epub 2024 Dec 26. Int J Antimicrob Agents. 2025. PMID: 39730229 Free PMC article. No abstract available.
Abstract
Background: Chloroquine and hydroxychloroquine have been found to be efficient on SARS-CoV-2, and reported to be efficient in Chinese COV-19 patients. We evaluate the effect of hydroxychloroquine on respiratory viral loads.
Patients and methods: French Confirmed COVID-19 patients were included in a single arm protocol from early March to March 16th, to receive 600mg of hydroxychloroquine daily and their viral load in nasopharyngeal swabs was tested daily in a hospital setting. Depending on their clinical presentation, azithromycin was added to the treatment. Untreated patients from another center and cases refusing the protocol were included as negative controls. Presence and absence of virus at Day6-post inclusion was considered the end point.
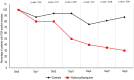
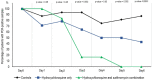
Results: Six patients were asymptomatic, 22 had upper respiratory tract infection symptoms and eight had lower respiratory tract infection symptoms. Twenty cases were treated in this study and showed a significant reduction of the viral carriage at D6-post inclusion compared to controls, and much lower average carrying duration than reported in the litterature for untreated patients. Azithromycin added to hydroxychloroquine was significantly more efficient for virus elimination.
Conclusion: Despite its small sample size, our survey shows that hydroxychloroquine treatment is significantly associated with viral load reduction/disappearance in COVID-19 patients and its effect is reinforced by azithromycin.
This article has been retracted: please see Elsevier Policy on Article Withdrawal (https://www.elsevier.com/locate/withdrawalpolicy). Concerns have been raised regarding this article, the substance of which relate to the articles' adherence to Elsevier's publishing ethics policies and the appropriate conduct of research involving human participants, as well as concerns raised by three of the authors themselves regarding the article's methodology and conclusions. Elsevier's Research Integrity and Publishing Ethics Team, in collaboration with the journal's co-owner, the International Society of Antimicrobial Chemotherapy (ISAC), and with guidance from an impartial field expert acting in the role of an independent Publishing Ethics Advisor, Dr. Jim Gray, Consultant Microbiologist at the Birmingham Children's and Women's Hospitals, U.K., conducted an investigation and determined that the below points constituted cause for retraction: • The journal has been unable to confirm whether any of the patients for this study were accrued before ethical approval had been obtained. The ethical approval dates for this article are stated as being 5th and 6th of March 2020 (ANSM and CPP respectively), while the article states that recruitment began in “early March”. The 17th author, Prof. Philippe Brouqui, has confirmed that the start date for patient accrual was 6th March 2020. The journal has not been able to establish whether all patients could have entered into the study in time for the data to have been analysed and included in the manuscript prior to its submission on the 20th March 2020, nor whether all patients were enrolled in the study upon admission as opposed to having been hospitalised for some time before starting the treatment described in the article. Additionally, the journal has not been able to establish whether there was equipoise between the study patients and the control patients. • The journal has not been able to establish whether the subjects in this study should have provided informed consent to receive azithromycin as part of the study. The journal has concluded that that there is reasonable cause to conclude that azithromycin was not considered standard care at the time of the study. The 17th author, Prof. Philippe Brouqui has attested that azithromycin treatment was not, at the time of the study, an experimental treatment but a possible treatment for, or preventative measure against, bacterial superinfections of viral pneumonia as described in section 2.4 of the article, and as such the treatment should be categorised as standard care that would not require informed consent. This does not fully address the journal's concerns around the use of azithromycin in the study. In section 3.1 of the article, it is stated that six patients received azithromycin to prevent (rather than treat) bacterial superinfection. All of these were amongst the patients who also received hydroxychloroquine (HCQ). None of the control patients are reported to have received azithromycin. This would indicate that only patients in the HCQ arm received azithromycin, all of whom were in one center. The recommendations for use of macrolides in France at the time the study was conducted indicate that azithromycin would not have been a logical agent to use as first-line prophylaxis against pneumonia due to the frequency of macrolide resistance amongst bacteria such as pneumococci. These two points suggest that azithromycin would not have been standard practice across southern France at the time the study was conducted and would have required informed consent. • Three of the authors of this article, Dr. Johan Courjon, Prof. Valérie Giordanengo, and Dr. Stéphane Honoré have contacted the journal to assert their opinion that they have concerns regarding the presentation and interpretation of results in this article and have stated they no longer wish to see their names associated with the article. • Author Prof. Valérie Giordanengo informed the journal that while the PCR tests administered in Nice were interpreted according to the recommendations of the national reference center, it is believed that those carried out in Marseille were not conducted using the same technique or not interpreted according to the same recommendations, which in her opinion would have resulted in a bias in the analysis of the data. This raises concerns as to whether the study was partially conducted counter to national guidelines at that time. The 17th author, Prof. Philippe Brouqui has attested that the PCR methodology was explained in reference 17 of the article. However, the article referred to by reference 17 describes several diagnostic approaches that were used (one PCR targeting the envelope protein only; another targeting the spike protein; and three commercially produced systems by QuantiNova, Biofire, and FTD). This reference does not clarify how the results were interpreted. It has also been noted during investigation of these concerns that only 76% (19/25) of patients were viral culture positive, resulting in uncertainty in the interpretation of PCR reports as has been raised by Prof. Giordanengo. As part of the investigation, the corresponding author was contacted and asked to provide an explanation for the above concerns. No response has been received within the deadline provided by the journal. Responses were received by the 3rd and 17th authors, Prof. Philippe Parola and Prof. Philippe Brouqui, respectively, and were reviewed as part of the investigation. These two authors, in addition to 1st author Dr. Philippe Gautret, 13th author Prof. Philippe Colson, and 15th author Prof. Bernard La Scola, disagreed with the retraction and dispute the grounds for it. Having followed due process and concluded the aforementioned investigation and based on the recommendation of Dr. Jim Gray acting in his capacity as independent Publishing Ethics Advisor, the co-owners of the journal (Elsevier and ISAC) have therefore taken the decision to retract the article.
Keywords: 2019-nCoV; Azithromycin; COVID-19; Clinical trial; Hydroxychloroquine; SARS-CoV-2.
Copyright © 2020. Published by Elsevier B.V.
Figures
Comment in
-
Does hydroxychloroquine prevent the transmission of COVID-19?Ann Rheum Dis. 2020 Jun;79(6):e60. doi: 10.1136/annrheumdis-2020-217501. Epub 2020 Apr 15. Ann Rheum Dis. 2020. PMID: 32295788 No abstract available.
-
Taking the Longer View of COVID-19.Oncologist. 2020 Jun;25(6):455-457. doi: 10.1634/theoncologist.2020-0313. Epub 2020 Apr 27. Oncologist. 2020. PMID: 32304334 Free PMC article.
-
The quality of the reported sample size calculation in clinical trials on COVID-19 patients indexed in PubMed.Eur J Intern Med. 2020 Jul;77:139-140. doi: 10.1016/j.ejim.2020.04.057. Epub 2020 Apr 30. Eur J Intern Med. 2020. PMID: 32414641 Free PMC article. No abstract available.
-
Pharmacist's perspective on HCQ treatment of COVID-19.Kaohsiung J Med Sci. 2020 Aug;36(8):660. doi: 10.1002/kjm2.12249. Epub 2020 Jun 12. Kaohsiung J Med Sci. 2020. PMID: 32533592 Free PMC article. No abstract available.
-
Hydroxychloroquine/ chloroquine as a treatment choice or prophylaxis for Covid-19 at the primary care level in developing countries: A Primum non Nocere dilemma.J Neurol Sci. 2020 Aug 15;415:116972. doi: 10.1016/j.jns.2020.116972. Epub 2020 Jun 3. J Neurol Sci. 2020. PMID: 32534369 Free PMC article.
-
Azithromycin and SARS-CoV-2 infection: Where we are now and where we are going.J Glob Antimicrob Resist. 2020 Sep;22:680-684. doi: 10.1016/j.jgar.2020.06.016. Epub 2020 Jul 1. J Glob Antimicrob Resist. 2020. PMID: 32622008 Free PMC article.
-
Benefit v. risk when using chloroquine in patients with severe COVID-19 disease.S Afr Med J. 2020 Apr 2;110(5):12903. doi: 10.7196/SAMJ.2020.v110i5.14761. S Afr Med J. 2020. PMID: 32657706 No abstract available.
-
Review of: "Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial Gautret et al 2010, DOI:10.1016/j.ijantimicag.2020.105949.Int J Antimicrob Agents. 2020 Jul;56(1):106063. doi: 10.1016/j.ijantimicag.2020.106063. Epub 2020 Jul 13. Int J Antimicrob Agents. 2020. PMID: 32674928 Free PMC article. No abstract available.
-
RE: "EARLY OUTPATIENT TREATMENT OF SYMPTOMATIC, HIGH-RISK COVID-19 PATIENTS THAT SHOULD BE RAMPED UP IMMEDIATELY AS KEY TO THE PANDEMIC CRISIS".Am J Epidemiol. 2020 Nov 2;189(11):1443-1444. doi: 10.1093/aje/kwaa151. Am J Epidemiol. 2020. PMID: 32685975 Free PMC article. No abstract available.
-
Apparent inefficacy of hydroxychloroquine combined with azithromycin on SARS-CoV-2 clearance in an incident cohort of geriatric patients with COVID-19.Travel Med Infect Dis. 2020 Sep-Oct;37:101826. doi: 10.1016/j.tmaid.2020.101826. Epub 2020 Jul 31. Travel Med Infect Dis. 2020. PMID: 32739472 Free PMC article. No abstract available.
-
Flexibilization of Science, Cognitive Biases, and the COVID-19 Pandemic.Mayo Clin Proc. 2020 Sep;95(9):1842-1844. doi: 10.1016/j.mayocp.2020.06.037. Mayo Clin Proc. 2020. PMID: 32861327 Free PMC article. No abstract available.
-
Análisis preliminar in silico de azitromicina con proteínas humanas relacionadas al SARS-CoV-2.Rev Peru Med Exp Salud Publica. 2020 Apr-Jun;37(2):383-384. doi: 10.17843/rpmesp.2020.372.5465. Epub 2020 Aug 28. Rev Peru Med Exp Salud Publica. 2020. PMID: 32876237 Spanish. No abstract available.
-
An Ounce of Prevention and a Pound of Cure: Randomized Clinical Trials of Therapeutics Against COVID-19 and an Assessment of Personal Protective Equipment and Distancing.Int J Radiat Oncol Biol Phys. 2020 Oct 1;108(2):333-336. doi: 10.1016/j.ijrobp.2020.08.001. Int J Radiat Oncol Biol Phys. 2020. PMID: 32890500 Free PMC article. No abstract available.
-
Management of Irrational Self-Purchase of Hydroxychloroquine During the COVID-19 Pandemic: Experiences From the Largest Healthcare System in Taiwan.J Patient Saf. 2021 Jan 1;17(1):e43-e44. doi: 10.1097/PTS.0000000000000795. J Patient Saf. 2021. PMID: 33074933 Free PMC article. No abstract available.
-
Disputes over the production and dissemination of misinformation in the time of COVID-19.Respir Med. 2021 Jun;182:106380. doi: 10.1016/j.rmed.2021.106380. Epub 2021 Mar 29. Respir Med. 2021. PMID: 33930690 Free PMC article.
-
Scientific image sleuth faces legal action for criticizing research papers.Nature. 2021 Jun;594(7861):17-18. doi: 10.1038/d41586-021-01430-z. Nature. 2021. PMID: 34045759 No abstract available.
Similar articles
-
Defining the optimum strategy for identifying adults and children with coeliac disease: systematic review and economic modelling.Health Technol Assess. 2022 Oct;26(44):1-310. doi: 10.3310/ZUCE8371. Health Technol Assess. 2022. PMID: 36321689 Free PMC article.
-
Depressing time: Waiting, melancholia, and the psychoanalytic practice of care.In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. PMID: 36137063 Free Books & Documents. Review.
-
Expert Witness.2024 Dec 7. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Dec 7. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 28613772 Free Books & Documents.
-
Ceftazidime with avibactam for treating severe aerobic Gram-negative bacterial infections: technology evaluation to inform a novel subscription-style payment model.Health Technol Assess. 2024 Oct;28(73):1-230. doi: 10.3310/YAPL9347. Health Technol Assess. 2024. PMID: 39487661 Free PMC article.
-
Antibody tests for identification of current and past infection with SARS-CoV-2.Cochrane Database Syst Rev. 2022 Nov 17;11(11):CD013652. doi: 10.1002/14651858.CD013652.pub2. Cochrane Database Syst Rev. 2022. PMID: 36394900 Free PMC article. Review.
Cited by
-
Clinical Features and Prognostic Factors of 245 Portuguese Patients Hospitalized With COVID-19.Cureus. 2021 Mar 4;13(3):e13687. doi: 10.7759/cureus.13687. Cureus. 2021. PMID: 33833912 Free PMC article.
-
The effect of 5-day course of hydroxychloroquine and azithromycin combination on QT interval in non-ICU COVID19(+) patients.J Electrocardiol. 2020 Sep-Oct;62:59-64. doi: 10.1016/j.jelectrocard.2020.08.008. Epub 2020 Aug 11. J Electrocardiol. 2020. PMID: 32827987 Free PMC article.
-
Repurposing Drugs, Ongoing Vaccine, and New Therapeutic Development Initiatives Against COVID-19.Front Pharmacol. 2020 Aug 19;11:1258. doi: 10.3389/fphar.2020.01258. eCollection 2020. Front Pharmacol. 2020. PMID: 32973505 Free PMC article. Review.
-
In vitro safety "clinical trial" of the cardiac liability of drug polytherapy.Clin Transl Sci. 2021 May;14(3):1155-1165. doi: 10.1111/cts.13038. Epub 2021 May 3. Clin Transl Sci. 2021. PMID: 33786981 Free PMC article.
-
COVID-19 and cardiovascular disease: from basic mechanisms to clinical perspectives.Nat Rev Cardiol. 2020 Sep;17(9):543-558. doi: 10.1038/s41569-020-0413-9. Epub 2020 Jul 20. Nat Rev Cardiol. 2020. PMID: 32690910 Free PMC article. Review.
References
-
- Lai C.C., Shih T.P., Ko W.C., Tang H.J., Hsueh P.R. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): The epidemic and the challenges. Int J Antimicrob Agents. 2020 Feb 17 doi: 10.1016/j.ijantimicag.2020.105924. [Epub ahead of print] - DOI - PMC - PubMed
-
- WHO Director-General's opening remarks at the media briefing on COVID-19 - 11 March 2020. [https://www.who.int/dg/speeches/detail/who-director-general-s-opening-re...]
-
- Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020 Feb 24 doi: 10.1001/jama.2020.2648. [Epub ahead of print] - DOI - PubMed
-
- Santé Publique France. Infection au nouveau Coronavirus (SARS-CoV-2), COVID-19, France et Monde [https://www.santepubliquefrance.fr/maladies-et-traumatismes/maladies-et-...]
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

