Once-weekly Somapacitan is Effective and Well Tolerated in Adults with GH Deficiency: A Randomized Phase 3 Trial
- PMID: 32022863
- PMCID: PMC7076631
- DOI: 10.1210/clinem/dgaa049
Once-weekly Somapacitan is Effective and Well Tolerated in Adults with GH Deficiency: A Randomized Phase 3 Trial
Abstract
Context: Growth hormone (GH) replacement requires daily GH injections, which is burdensome for some adult patients with GH deficiency (AGHD).
Objective: To demonstrate efficacy and safety of somapacitan, a once-weekly reversible albumin-binding GH derivative, versus placebo in AGHD.
Design: Randomized, parallel-group, placebo-controlled (double-blind) and active-controlled (open-label) phase 3 trial, REAL 1 (NCT02229851).
Setting: Clinics in 17 countries.
Patients: Treatment-naïve patients with AGHD (n = 301 main study period, 272 extension period); 257 patients completed the trial.
Interventions: Patients were randomized 2:2:1 to once-weekly somapacitan, daily GH, or once-weekly placebo for 34 weeks (main period). During the 52-week extension period, patients continued treatment with somapacitan or daily GH.
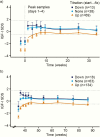
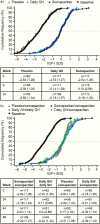
Main outcome measures: Body composition measured using dual-energy x-ray absorptiometry (DXA). The primary endpoint was change in truncal fat percentage to week 34. Insulin-like growth factor 1 (IGF-I) standard deviation score (SDS) values were used to dose titrate.
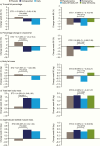
Results: At 34 weeks, somapacitan significantly reduced truncal fat percentage (estimated difference: -1.53% [-2.68; -0.38]; P = 0.0090), demonstrating superiority compared with placebo, and it improved other body composition parameters (including visceral fat and lean body mass) and IGF-I SDS. At 86 weeks, improvements were maintained with both somapacitan and daily GH. Somapacitan was well tolerated, with similar adverse events (including injection-site reactions) compared with daily GH.
Conclusions: In AGHD patients, somapacitan administered once weekly demonstrated superiority over placebo, and the overall treatment effects and safety of somapacitan were in accordance with known effects and safety of GH replacement for up to 86 weeks of treatment. Somapacitan may provide an effective alternative to daily GH in AGHD. A short visual summary of our work is available (1).
Keywords: REAL 1; adult growth hormone deficiency; body composition; hypopituitarism; long-acting growth hormone; somapacitan.
© Endocrine Society 2020.
Figures



Similar articles
-
Once-Weekly Somapacitan vs Daily GH in Children With GH Deficiency: Results From a Randomized Phase 2 Trial.J Clin Endocrinol Metab. 2020 Apr 1;105(4):e1847-61. doi: 10.1210/clinem/dgz310. J Clin Endocrinol Metab. 2020. PMID: 31917835 Free PMC article. Clinical Trial.
-
Similar safety and efficacy in previously treated adults with growth hormone deficiency randomized to once-weekly somapacitan or daily growth hormone.Clin Endocrinol (Oxf). 2020 Nov;93(5):620-628. doi: 10.1111/cen.14273. Epub 2020 Aug 14. Clin Endocrinol (Oxf). 2020. PMID: 32603494 Free PMC article. Clinical Trial.
-
Safety and convenience of once-weekly somapacitan in adult GH deficiency: a 26-week randomized, controlled trial.Eur J Endocrinol. 2018 May;178(5):491-499. doi: 10.1530/EJE-17-1073. Epub 2018 Mar 2. Eur J Endocrinol. 2018. PMID: 29500310 Free PMC article. Clinical Trial.
-
Utilizing Somapacitan, a Long-acting Growth Hormone Formulation, for the Treatment of Adult Growth Hormone Deficiency: A Guide for Clinicians.Endocr Pract. 2024 Oct;30(10):1003-1010. doi: 10.1016/j.eprac.2024.07.003. Epub 2024 Jul 9. Endocr Pract. 2024. PMID: 38992799 Review.
-
Somapacitan: a long-acting growth hormone derivative for treatment of growth hormone deficiency.Drugs Today (Barc). 2022 Oct;58(10):509-517. doi: 10.1358/dot.2022.58.10.3419556. Drugs Today (Barc). 2022. PMID: 36305544 Review.
Cited by
-
The Use of IGF-I to Monitor Long-Acting Growth Hormone Therapy-Timing is an Art….J Clin Endocrinol Metab. 2021 Apr 23;106(5):e2367-e2369. doi: 10.1210/clinem/dgab016. J Clin Endocrinol Metab. 2021. PMID: 33524146 Free PMC article. No abstract available.
-
Model-Based Analysis of IGF-I Response, Dosing, and Monitoring for Once-Weekly Somapacitan in Children With GH Deficiency.J Endocr Soc. 2023 Sep 11;7(11):bvad115. doi: 10.1210/jendso/bvad115. eCollection 2023 Oct 9. J Endocr Soc. 2023. PMID: 37818403 Free PMC article.
-
Effective GH Replacement With Once-weekly Somapacitan vs Daily GH in Children with GHD: 3-year Results From REAL 3.J Clin Endocrinol Metab. 2022 Apr 19;107(5):1357-1367. doi: 10.1210/clinem/dgab928. J Clin Endocrinol Metab. 2022. PMID: 34964458 Free PMC article. Clinical Trial.
-
Patient preferences for growth hormone treatment in Japanese children.Pediatr Int. 2021 Oct;63(10):1185-1191. doi: 10.1111/ped.14760. Epub 2021 Aug 25. Pediatr Int. 2021. PMID: 33930225 Free PMC article.
-
Guidance for the treatment of adult growth hormone deficiency with somapacitan, a long-acting growth hormone preparation.Front Endocrinol (Lausanne). 2022 Dec 23;13:1040046. doi: 10.3389/fendo.2022.1040046. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 36619571 Free PMC article. Review.
References
-
- Johannsson G, Gordon MB, Højby Rasmussen M, et al. Animated summary. https://nn-product.videomarketingplatform.co/secret/61296995/c7299d39259...
-
- Jørgensen JOL, Juul A. THERAPY OF ENDOCRINE DISEASE: Growth hormone replacement therapy in adults: 30 years of personal clinical experience. Eur J Endocrinol. 2018;179(1):R47–R56. - PubMed
-
- Melmed S. Pathogenesis and diagnosis of growth hormone deficiency in adults. N Engl J Med. 2019;380(26):2551–2562. - PubMed
-
- Stochholm K, Gravholt CH, Laursen T, et al. . Mortality and GH deficiency: a nationwide study. Eur J Endocrinol. 2007;157(1):9–18. - PubMed
-
- Fleseriu M, Hashim IA, Karavitaki N, et al. . Hormonal replacement in hypopituitarism in adults: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2016;101(11):3888–3921. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

