A 3-week nonalcoholic steatohepatitis mouse model shows elafibranor benefits on hepatic inflammation and cell death
- PMID: 31981449
- PMCID: PMC7214663
- DOI: 10.1111/cts.12735
A 3-week nonalcoholic steatohepatitis mouse model shows elafibranor benefits on hepatic inflammation and cell death
Abstract
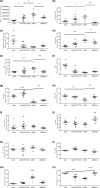
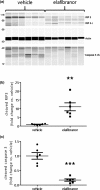
The long duration of animal models represents a clear limitation to quickly evaluate the efficacy of drugs targeting nonalcoholic steatohepatitis (NASH). We, therefore, developed a rapid mouse model of liver inflammation (i.e., the mouse fed a high-fat/high-cholesterol diet, where cyclodextrin is co-administered to favor hepatic cholesterol loading, liver inflammation, and NASH within 3 weeks), and evaluated the effects of the dual peroxisome proliferator-activated receptor alpha/delta agonist elafibranor (ELA). C57BL6/J mice were fed a 60% high-fat, 1.25% cholesterol, and 0.5% cholic acid diet with 2% cyclodextrin in drinking water (HFCC/CDX diet) for 3 weeks. After 1 week of the diet, mice were treated orally with vehicle or ELA 20 mg/kg q.d. for 2 weeks. Compared with vehicle, ELA markedly reduced liver lipids and nonalcoholic fatty liver disease activity scoring, through steatosis, inflammation, and fibrosis (all P < 0.01 vs. vehicle). Flow cytometry analysis showed that ELA significantly improved the HFCC/CDX diet-induced liver inflammation by preventing the increase in total number of immune cells (CD45+), Kupffer cells, dendritic cells, and monocytes population, as well as the reduction in natural killer and natural killer T cells, and by blocking conversion of T cells in regulatory T cells. ELA did not alter pyroptosis (Gasdermin D), but significantly reduced necroptosis (cleaved RIP3) and apoptosis (cleaved caspase 3) in the liver. In conclusion, ELA showed strong benefits on NASH, including improvement in hepatic inflammation, necroptosis, and apoptosis in the 3-week NASH mouse. This preclinical model will be useful to rapidly detect the effects of novel drugs targeting NASH.
© 2019 The Authors. Clinical and Translational Science published by Wiley Periodicals, Inc. on behalf of the American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
F.B., L.B., E.B., M.Q., and T.S. are employees of Physiogenex. F.B., R.B., and T.S. declare shares in Physiogenex. All other authors declared no competing interests for this work.
Figures


Similar articles
-
Metabolic and hepatic effects of liraglutide, obeticholic acid and elafibranor in diet-induced obese mouse models of biopsy-confirmed nonalcoholic steatohepatitis.World J Gastroenterol. 2018 Jan 14;24(2):179-194. doi: 10.3748/wjg.v24.i2.179. World J Gastroenterol. 2018. PMID: 29375204 Free PMC article.
-
Liraglutide improves hepatic steatosis and metabolic dysfunctions in a 3-week dietary mouse model of nonalcoholic steatohepatitis.Am J Physiol Gastrointest Liver Physiol. 2019 Oct 1;317(4):G508-G517. doi: 10.1152/ajpgi.00139.2019. Epub 2019 Aug 28. Am J Physiol Gastrointest Liver Physiol. 2019. PMID: 31460789
-
Elafibranor improves diet-induced nonalcoholic steatohepatitis associated with heart failure with preserved ejection fraction in Golden Syrian hamsters.Metabolism. 2021 Apr;117:154707. doi: 10.1016/j.metabol.2021.154707. Epub 2021 Jan 11. Metabolism. 2021. PMID: 33444606
-
Elafibranor: a potential drug for the treatment of nonalcoholic steatohepatitis (NASH).Expert Opin Investig Drugs. 2020 Feb;29(2):117-123. doi: 10.1080/13543784.2020.1668375. Epub 2019 Sep 25. Expert Opin Investig Drugs. 2020. PMID: 31523999 Review.
-
Anti-NASH Drug Development Hitches a Lift on PPAR Agonism.Cells. 2019 Dec 21;9(1):37. doi: 10.3390/cells9010037. Cells. 2019. PMID: 31877771 Free PMC article. Review.
Cited by
-
Metabolic Spectrum of Liver Failure in Type 2 Diabetes and Obesity: From NAFLD to NASH to HCC.Int J Mol Sci. 2021 Apr 26;22(9):4495. doi: 10.3390/ijms22094495. Int J Mol Sci. 2021. PMID: 33925827 Free PMC article. Review.
-
Peroxisome Proliferator-Activated Receptors and Their Novel Ligands as Candidates for the Treatment of Non-Alcoholic Fatty Liver Disease.Cells. 2020 Jul 8;9(7):1638. doi: 10.3390/cells9071638. Cells. 2020. PMID: 32650421 Free PMC article. Review.
-
Peroxisome Proliferator-Activated Receptors Regulate Hepatic Immunity and Assist in the Treatment of Primary Biliary Cholangitis.Front Immunol. 2022 Jul 8;13:940688. doi: 10.3389/fimmu.2022.940688. eCollection 2022. Front Immunol. 2022. PMID: 35880178 Free PMC article. Review.
-
Elafibranor alleviates alcohol-related liver fibrosis by restoring intestinal barrier function.World J Gastroenterol. 2024 Nov 21;30(43):4660-4668. doi: 10.3748/wjg.v30.i43.4660. World J Gastroenterol. 2024. PMID: 39575408 Free PMC article.
-
High-cholesterol diet in combination with hydroxypropyl-beta-cyclodextrin induces NASH-like disorders in the liver of rats.Physiol Res. 2023 Jul 14;72(3):371-382. doi: 10.33549/physiolres.934981. Physiol Res. 2023. PMID: 37449749 Free PMC article.
References
-
- Yonoussi, Z.M. Non‐alcoholic fatty liver disease – a global public health perspective. J. Hepatol. 70, 531–544 (2019). - PubMed
-
- Samji, N.S. et al Liver transplantation for nonalcoholic steatohepatitis: pathophysiology of recurrence and clinical challenges. Dig. Dis. Sci. 64, 3413–3430 (2019). - PubMed
-
- Arguello, G. , Balboa, E. , Arrese, M. & Zanlungo, S. Recent insights on the role of cholesterol in non‐alcoholic fatty liver disease. Biochim. Biophys. Acta 1852, 1765–1778 (2015). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

