Liver Glycogen Phosphorylase Deficiency Leads to Profibrogenic Phenotype in a Murine Model of Glycogen Storage Disease Type VI
- PMID: 31701076
- PMCID: PMC6824077
- DOI: 10.1002/hep4.1426
Liver Glycogen Phosphorylase Deficiency Leads to Profibrogenic Phenotype in a Murine Model of Glycogen Storage Disease Type VI
Abstract
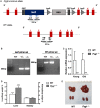
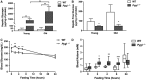
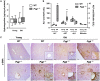
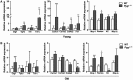
Mutations in the liver glycogen phosphorylase (Pygl) gene are associated with the diagnosis of glycogen storage disease type VI (GSD-VI). To understand the pathogenesis of GSD-VI, we generated a mouse model with Pygl deficiency (Pygl -/-). Pygl -/- mice exhibit hepatomegaly, excessive hepatic glycogen accumulation, and low hepatic free glucose along with lower fasting blood glucose levels and elevated blood ketone bodies. Hepatic glycogen accumulation in Pygl -/- mice increases with age. Masson's trichrome and picrosirius red staining revealed minimal to mild collagen deposition in periportal, subcapsular, and/or perisinusoidal areas in the livers of old Pygl -/- mice (>40 weeks). Consistently, immunohistochemical analysis showed the number of cells positive for alpha smooth muscle actin (α-SMA), a marker of activated hepatic stellate cells, was increased in the livers of old Pygl -/- mice compared with those of age-matched wild-type (WT) mice. Furthermore, old Pygl -/- mice had inflammatory infiltrates associated with hepatic vessels in their livers along with up-regulated hepatic messenger RNA levels of C-C chemokine ligand 5 (Ccl5/Rantes) and monocyte chemoattractant protein 1 (Mcp-1), indicating inflammation, while age-matched WT mice did not. Serum levels of aspartate aminotransferase and alanine aminotransferase were elevated in old Pygl -/- mice, indicating liver damage. Conclusion: Pygl deficiency results in progressive accumulation of hepatic glycogen with age and liver damage, inflammation, and collagen deposition, which can increase the risk of liver fibrosis. Collectively, the Pygl-deficient mouse recapitulates clinical features in patients with GSD-VI and provides a model to elucidate the mechanisms underlying hepatic complications associated with defective glycogen metabolism.
© 2019 The Authors. Hepatology Communications published by Wiley Periodicals, Inc., on behalf of the American Association for the Study of Liver Diseases.
Figures


Similar articles
-
Novel PYGL mutations in Chinese children leading to glycogen storage disease type VI: two case reports.BMC Med Genet. 2020 Apr 8;21(1):74. doi: 10.1186/s12881-020-01010-4. BMC Med Genet. 2020. PMID: 32268899 Free PMC article.
-
A Mouse Model of Glycogen Storage Disease Type IX-Beta: A Role for Phkb in Glycogenolysis.Int J Mol Sci. 2022 Sep 1;23(17):9944. doi: 10.3390/ijms23179944. Int J Mol Sci. 2022. PMID: 36077341 Free PMC article.
-
The Phenotypic and Genetic Spectrum of Glycogen Storage Disease Type VI.Genes (Basel). 2021 Aug 3;12(8):1205. doi: 10.3390/genes12081205. Genes (Basel). 2021. PMID: 34440378 Free PMC article. Review.
-
Hepatic ChREBP orchestrates intrahepatic carbohydrate metabolism to limit hepatic glucose 6-phosphate and glycogen accumulation in a mouse model for acute Glycogen Storage Disease type Ib.Mol Metab. 2024 Jan;79:101838. doi: 10.1016/j.molmet.2023.101838. Epub 2023 Nov 22. Mol Metab. 2024. PMID: 37995884 Free PMC article.
-
Glycogen storage disease type VI with a novel PYGL mutation: Two case reports and literature review.Medicine (Baltimore). 2021 Apr 23;100(16):e25520. doi: 10.1097/MD.0000000000025520. Medicine (Baltimore). 2021. PMID: 33879691 Free PMC article. Review.
Cited by
-
Unveiling the role of PYGB in pancreatic cancer: a novel diagnostic biomarker and gene therapy target.J Cancer Res Clin Oncol. 2024 Mar 14;150(3):127. doi: 10.1007/s00432-024-05644-2. J Cancer Res Clin Oncol. 2024. PMID: 38483604 Free PMC article.
-
Novel PYGL mutations in Chinese children leading to glycogen storage disease type VI: two case reports.BMC Med Genet. 2020 Apr 8;21(1):74. doi: 10.1186/s12881-020-01010-4. BMC Med Genet. 2020. PMID: 32268899 Free PMC article.
-
Preclinical Research in Glycogen Storage Diseases: A Comprehensive Review of Current Animal Models.Int J Mol Sci. 2020 Dec 17;21(24):9621. doi: 10.3390/ijms21249621. Int J Mol Sci. 2020. PMID: 33348688 Free PMC article. Review.
-
Integrated liver proteomics and metabolomics identify metabolic pathways affected by pantothenic acid deficiency in Pekin ducks.Anim Nutr. 2022 Jun 21;11:1-14. doi: 10.1016/j.aninu.2022.03.008. eCollection 2022 Dec. Anim Nutr. 2022. PMID: 35950191 Free PMC article.
-
Investigating Neuron Degeneration in Huntington's Disease Using RNA-Seq Based Transcriptome Study.Genes (Basel). 2023 Sep 14;14(9):1801. doi: 10.3390/genes14091801. Genes (Basel). 2023. PMID: 37761940 Free PMC article.
References
-
- Roscher A, Patel J, Hewson S, Nagy L, Feigenbaum A, Kronick J, et al. The natural history of glycogen storage disease types VI and IX: long‐term outcome from the largest metabolic center in Canada. Mol Genet Metab 2014;113:171‐176. - PubMed
-
- Sutherland EW Jr, Wosilait WD. Inactivation and activation of liver phosphorylase. Nature 1955;175:169‐170. - PubMed
-
- Tang NL, Hui J, Young E, Worthington V, To KF, Cheung KL, et al. A novel mutation (G233D) in the glycogen phosphorylase gene in a patient with hepatic glycogen storage disease and residual enzyme activity. Mol Genet Metab 2003;79:142‐145. - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

