APOLLO-1: a randomized placebo and active-controlled phase III study investigating oliceridine (TRV130), a G protein-biased ligand at the µ-opioid receptor, for management of moderate-to-severe acute pain following bunionectomy
- PMID: 30881102
- PMCID: PMC6417853
- DOI: 10.2147/JPR.S171013
APOLLO-1: a randomized placebo and active-controlled phase III study investigating oliceridine (TRV130), a G protein-biased ligand at the µ-opioid receptor, for management of moderate-to-severe acute pain following bunionectomy
Abstract
Purpose: Oliceridine is a novel G protein-biased µ-opioid receptor agonist designed to provide intravenous (IV) analgesia with a lower risk of opioid-related adverse events (ORAEs) than conventional opioids.
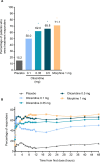
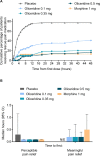
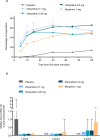
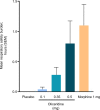
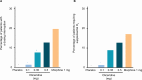
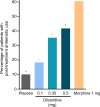
Patients and methods: APOLLO-1 (NCT02815709) was a phase III, double-blind, randomized trial in patients with moderate-to-severe pain following bunionectomy. Patients received a loading dose of either placebo, oliceridine (1.5 mg), or morphine (4 mg), followed by demand doses via patient-controlled analgesia (0.1, 0.35, or 0.5 mg oliceridine, 1 mg morphine, or placebo). The primary endpoint compared the proportion of treatment responders through 48 hours for oliceridine regimens and placebo. Secondary outcomes included a composite measure of respiratory safety burden (RSB, representing the cumulative duration of respiratory safety events) and the proportion of treatment responders vs morphine.
Results: Effective analgesia was observed for all oliceridine regimens, with responder rates of 50%, 62%, and 65.8% in the 0.1 mg, 0.35 mg, and 0.5 mg regimens, respectively (all P<0.0001 vs placebo [15.2%]; 0.35 mg and 0.5 mg non-inferior to morphine). RSB showed a dose-dependent increase across oliceridine regimens (mean hours [SD]: 0.1 mg: 0.04 [0.33]; 0.35 mg: 0.28 [1.11]; 0.5 mg: 0.8 [3.33]; placebo: 0 [0]), but none were statistically different from morphine (1.1 [3.03]). Gastrointestinal adverse events also increased in a dose-dependent manner in oliceridine regimens (0.1 mg: 40.8%; 0.35 mg: 59.5%; 0.5 mg: 70.9%; placebo: 24.1%; morphine: 72.4%). The odds ratio for rescue antiemetic use was significantly lower for oliceridine regimens compared to morphine (P<0.05).
Conclusion: Oliceridine is a novel and effective IV analgesic providing rapid analgesia for the relief of moderate-to-severe acute postoperative pain compared to placebo. Additionally, it has a favorable safety and tolerability profile with regard to respiratory and gastrointestinal adverse effects compared to morphine, and may provide a new treatment option for patients with moderate-to-severe postoperative pain where an IV opioid is required.
Keywords: analgesia; clinical trial; orthopedic surgery; patient controlled; postoperative.
Conflict of interest statement
Disclosure David A Burt, Emily Cook, David G Soergel, and Franck Skobieranda were full-time employees and stockholders of Trevena Inc. at the time the research was conducted and manuscript was under preparation. Neil Singla is founder and CEO of Lotus Clinical Research, LLC, an analgesic Clinical Research Organization and research site; in this capacity he has served as a consultant and provided clinical trial services for Trevena Inc. Eugene R Viscusi has served as a consultant to Trevena Inc. The authors report no other conflicts of interest in this work.
Figures
Similar articles
-
APOLLO-2: A Randomized, Placebo and Active-Controlled Phase III Study Investigating Oliceridine (TRV130), a G Protein-Biased Ligand at the μ-Opioid Receptor, for Management of Moderate to Severe Acute Pain Following Abdominoplasty.Pain Pract. 2019 Sep;19(7):715-731. doi: 10.1111/papr.12801. Epub 2019 Jun 24. Pain Pract. 2019. PMID: 31162798 Free PMC article. Clinical Trial.
-
A randomized, Phase IIb study investigating oliceridine (TRV130), a novel µ-receptor G-protein pathway selective (μ-GPS) modulator, for the management of moderate to severe acute pain following abdominoplasty.J Pain Res. 2017 Oct 6;10:2413-2424. doi: 10.2147/JPR.S137952. eCollection 2017. J Pain Res. 2017. PMID: 29062240 Free PMC article.
-
Oliceridine is Associated with Reduced Risk of Vomiting and Need for Rescue Antiemetics Compared to Morphine: Exploratory Analysis from Two Phase 3 Randomized Placebo and Active Controlled Trials.Pain Ther. 2021 Jun;10(1):401-413. doi: 10.1007/s40122-020-00216-x. Epub 2020 Nov 18. Pain Ther. 2021. PMID: 33210266 Free PMC article.
-
Oliceridine: A Novel Drug for the Management of Moderate to Severe Acute Pain - A Review of Current Evidence.J Pain Res. 2021 Apr 14;14:969-979. doi: 10.2147/JPR.S278279. eCollection 2021. J Pain Res. 2021. PMID: 33889018 Free PMC article. Review.
-
A critical review of oliceridine injection as an IV opioid analgesic for the management of severe acute pain.Expert Rev Neurother. 2022 Jun;22(6):419-426. doi: 10.1080/14737175.2022.2072731. Epub 2022 May 17. Expert Rev Neurother. 2022. PMID: 35502668 Review.
Cited by
-
Novel Opioids in the Setting of Acute Postoperative Pain: A Narrative Review.Pharmaceuticals (Basel). 2023 Dec 25;17(1):29. doi: 10.3390/ph17010029. Pharmaceuticals (Basel). 2023. PMID: 38256863 Free PMC article. Review.
-
Oliceridine and its potential to revolutionize GI endoscopy sedation.Saudi J Anaesth. 2020 Jul-Sep;14(3):349-354. doi: 10.4103/sja.SJA_813_19. Epub 2020 May 30. Saudi J Anaesth. 2020. PMID: 32934628 Free PMC article. Review.
-
Bibliometric and Visualized Analyses of Research Studies on Different Analgesics in the Treatment of Orthopedic Postoperative Pain.Pain Res Manag. 2022 Feb 24;2022:6835219. doi: 10.1155/2022/6835219. eCollection 2022. Pain Res Manag. 2022. PMID: 35251417 Free PMC article.
-
The Oxford Catalogue of Opioids: A systematic synthesis of opioid drug names and their pharmacology.Br J Clin Pharmacol. 2021 Oct;87(10):3790-3812. doi: 10.1111/bcp.14786. Epub 2021 Mar 20. Br J Clin Pharmacol. 2021. PMID: 33608948 Free PMC article.
-
Low Incidence of Postoperative Respiratory Depression with Oliceridine Compared to Morphine: A Retrospective Chart Analysis.Pain Res Manag. 2020 Oct 24;2020:7492865. doi: 10.1155/2020/7492865. eCollection 2020. Pain Res Manag. 2020. PMID: 33163127 Free PMC article.
References
-
- National Pharmaceutical Council (NPC) Pain: current understanding of assessment, management, and treatments. Reston, VA: NPC, Joint Commission on Accreditation of Health Care Organization; 2001.
-
- Benyamin R, Trescot AM, Datta S, et al. Opioid complications and side effects. Pain Physician. 2008;11(2 Suppl):S105–S120. - PubMed
-
- Wheeler M, Oderda GM, Ashburn MA, Lipman AG. Adverse events associated with postoperative opioid analgesia: a systematic review. J Pain. 2002;3(3):159–180. - PubMed
-
- Swegle JM, Logemann C. Management of common opioid-induced adverse effects. Am Fam Physician. 2006;74(8):1347–1354. - PubMed
LinkOut - more resources
Full Text Sources

