Randomized trial of add-on triheptanoin vs medium chain triglycerides in adults with refractory epilepsy
- PMID: 30868125
- PMCID: PMC6398112
- DOI: 10.1002/epi4.12308
Randomized trial of add-on triheptanoin vs medium chain triglycerides in adults with refractory epilepsy
Abstract
Objective: To investigate the feasibility, safety, and tolerability of add-on treatment of the triglycerides of heptanoate (triheptanoin) vs the triglycerides of octanoate and decanoate (medium chain triglycerides [MCTs]) in adults with treatment-refractory epilepsy.
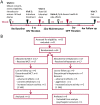
Methods: After an 8-week prospective baseline period, people with drug-resistant epilepsy were randomized in a double-blind fashion to receive triheptanoin or MCTs. Treatment was titrated over 3 weeks to a maximum of 100 mL/d to be distributed over 3 meals and mixed into food, followed by 12-week maintenance before tapering. The primary aims were to assess the following: (a) safety by comparing the number of intervention-related adverse events with triheptanoin vs MCT treatment and (b) adherence, measured as a percentage of the prescribed treatment doses taken.
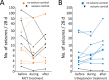
Results: Thirty-four people were randomized (17 to MCT and 17 to triheptanoin). There were no differences regarding (a) the number of participants completing the study (11 vs 9 participants), (b) the time until withdrawal, (c) the total number of adverse events or those potentially related to treatment, (d) median doses of oils taken (59 vs 55 mL/d, P = 0.59), or (e) change in seizure frequency (54% vs 102%, P = 0.13). Please note that people with focal unaware seizures were underrepresented in the triheptanoin treatment arm (P = 0.04). The most common adverse events were gastrointestinal disturbances (47% and 62.5% of participants). Five people taking on average 0.73 mL/kg body weight MCTs (0.64 mL/kg median) and one person taking 0.59 mL/kg triheptanoin showed >50% reduction in seizure frequency, specifically focal unaware seizures.
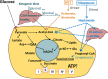
Significance: Add-on treatment with MCTs or triheptanoin was feasible, safe, and tolerated for 12 weeks in two-thirds of people with treatment-resistant epilepsy. Our results indicate a protective effect of MCTs on focal unaware seizures. This warrants further study.
Keywords: TCA cycle; anaplerosis; focal unaware seizure; medium chain triglyceride.
Conflict of interest statement
The study was supported by the Epilepsy Foundation New Therapy Development Program, Parents Against Childhood Epilepsy and Uniquest Pty. Ltd. Dr. Borges filed for a patent regarding triheptanoin in epilepsy and received a license fee payment and research support from Ultragenyx Pharmaceuticals Inc. Research support was also received from Epilepsy Foundation New Therapy Development Program, Parents Against Childhood Epilepsy, Uniquest Pty. Ltd., National Health and Medical Research Council (NHMRC), Motor Neurone Disease Research Institute Australia, and funding from the Brain Foundation (Australia). Dr. Terence J O'Brien reports grants and personal fees from UCB Pharma, Eisai, and Zynerba Pharmaceuticals, outside the submitted work. He has also received research grants from the NHMRC, National Institute of Neurological Disorders and Stroke (NINDS), and the RMH Neuroscience Foundation. Dr. Kwan/his institution received speaker or consultancy fees and/or research grants from Eisai, GlaxoSmithKline, Johnson & Johnson, Pfizer, and UCB Pharma. He is supported by the Medical Research Future Fund Practitioner Fellowship. He has received research grants from the National Health and Medical Research Council of Australia, the Australian Research Council, the US National Institutes of Health, Hong Kong Research Grants Council, Innovation and Technology Fund, Health and Health Services Research Fund, and Health and Medical Research Fund. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Figures
Similar articles
-
Open-label long-term treatment of add-on triheptanoin in adults with drug-resistant epilepsy.Epilepsia Open. 2020 Apr 12;5(2):230-239. doi: 10.1002/epi4.12391. eCollection 2020 Jun. Epilepsia Open. 2020. PMID: 32524048 Free PMC article.
-
A randomized, double-blind trial of triheptanoin for drug-resistant epilepsy in glucose transporter 1 deficiency syndrome.Epilepsia. 2022 Jul;63(7):1748-1760. doi: 10.1111/epi.17263. Epub 2022 May 21. Epilepsia. 2022. PMID: 35441706 Free PMC article. Clinical Trial.
-
A pilot study of add-on oral triheptanoin treatment for children with medically refractory epilepsy.Eur J Paediatr Neurol. 2018 Nov;22(6):1074-1080. doi: 10.1016/j.ejpn.2018.07.014. Epub 2018 Aug 7. Eur J Paediatr Neurol. 2018. PMID: 30126760 Clinical Trial.
-
Felbamate add-on therapy for drug-resistant focal epilepsy.Cochrane Database Syst Rev. 2022 Aug 1;8(8):CD008295. doi: 10.1002/14651858.CD008295.pub6. Cochrane Database Syst Rev. 2022. PMID: 35914010 Free PMC article. Review.
-
Brivaracetam add-on therapy for drug-resistant epilepsy.Cochrane Database Syst Rev. 2022 Mar 14;3(3):CD011501. doi: 10.1002/14651858.CD011501.pub3. Cochrane Database Syst Rev. 2022. PMID: 35285519 Free PMC article. Review.
Cited by
-
Fats, Friends or Foes: Investigating the Role of Short- and Medium-Chain Fatty Acids in Alzheimer's Disease.Biomedicines. 2022 Nov 1;10(11):2778. doi: 10.3390/biomedicines10112778. Biomedicines. 2022. PMID: 36359298 Free PMC article. Review.
-
Triheptanoin Mitigates Brain ATP Depletion and Mitochondrial Dysfunction in a Mouse Model of Alzheimer's Disease.J Alzheimers Dis. 2020;78(1):425-437. doi: 10.3233/JAD-200594. J Alzheimers Dis. 2020. PMID: 33016909 Free PMC article.
-
Astrocyte metabolism of the medium-chain fatty acids octanoic acid and decanoic acid promotes GABA synthesis in neurons via elevated glutamine supply.Mol Brain. 2021 Sep 3;14(1):132. doi: 10.1186/s13041-021-00842-2. Mol Brain. 2021. PMID: 34479615 Free PMC article.
-
Triheptanoin: First Approval.Drugs. 2020 Oct;80(15):1595-1600. doi: 10.1007/s40265-020-01399-5. Drugs. 2020. PMID: 32897506 Free PMC article. Review.
-
Updates on the ketogenic diet therapy for pediatric epilepsy.Biomed J. 2022 Feb;45(1):19-26. doi: 10.1016/j.bj.2021.11.003. Epub 2021 Nov 19. Biomed J. 2022. PMID: 34808422 Free PMC article. Review.
References
-
- Kuhl DE, Engel J Jr, Phelps ME, et al. Epileptic patterns of local cerebral metabolism and perfusion in humans determined by emission computed tomography of 18FDG and 13NH3. Ann Neurol. 1980;8:348–60. - PubMed
-
- Dube C, Boyet S, Marescaux C, et al. Relationship between neuronal loss and interictal glucose metabolism during the chronic phase of the lithium‐pilocarpine model of epilepsy in the immature and adult rat. Exp Neurol. 2001;167:227–41. - PubMed
LinkOut - more resources
Full Text Sources

