Quality assessment of controlled clinical trials published in Orthopaedics and Traumatology journals in Spanish: An observational study through handsearching and evidence mapping
- PMID: 30302249
- PMCID: PMC6170956
- DOI: 10.1177/2050312118801710
Quality assessment of controlled clinical trials published in Orthopaedics and Traumatology journals in Spanish: An observational study through handsearching and evidence mapping
Abstract
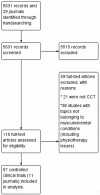
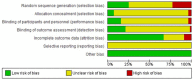
Few Orthopaedics and Traumatology journals from Latin America and Spain are indexed in major databases; controlled clinical trials published in these journals cannot be exhaustively retrieved using electronic literature searches. We aimed to identify, describe and assess the quality of controlled clinical trials published in Orthopaedics and Traumatology journals from Latin America and Spain through handsearching and evidence mapping methods. We identified controlled clinical trials published in eligible Orthopaedics/Traumatology journals in Spanish until July 2017 by handsearching. Data were extracted for controlled clinical trials main characteristics and the Cochrane risk of bias tool was used to assess the controlled clinical trials methodological quality. In addition, we mapped the main findings of these trials. As a result, we assessed 5631 references in 29 eligible journals of which 57 were controlled clinical trials (1.0%). Controlled clinical trials were published between 1995 and 2017 at a rate of 2.5 per year. Journals from Spain and Mexico published around 63% of the controlled clinical trials identified. The median sample size of patients enrolled was 60 (range = 30-300 participants). About conditions assessed, 38.5% of controlled clinical trials assessed issues related to knee conditions, 15.7% about hip and 10.5% about trauma or spine. The risk of bias domains most affected was selective reporting bias and random sequence generation. In addition, only two and seven trials had low risk of bias in all items related to participant/personnel and outcome assessment blindings, respectively. More than 40% of studies did not report differences on benefits/harms between the interventions assessed. As a conclusion, the number of controlled clinical trials published in Orthopaedics/Traumatology journals from Latin America and Spain is low. These controlled clinical trials had important methodological shortcomings and were judged as unclear or high risk of bias. These trials are now available in CENTRAL for their potential inclusion in systematic reviews and other documents of synthesis.
Keywords: Orthopaedics and Traumatology; controlled clinical trials; evidence mapping; handsearching; systematic review.
Conflict of interest statement
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures



Similar articles
-
Identification and description of controlled clinical trials published in Physiotherapy journals in Spain.J Eval Clin Pract. 2017 Feb;23(1):29-36. doi: 10.1111/jep.12441. Epub 2015 Sep 14. J Eval Clin Pract. 2017. PMID: 26370597
-
Identification and description of controlled clinical trials in Spanish language dental journals.Health Info Libr J. 2018 Sep;35(3):192-201. doi: 10.1111/hir.12214. Epub 2018 Apr 10. Health Info Libr J. 2018. PMID: 29635855
-
Handsearching versus electronic searching to identify reports of randomized trials.Cochrane Database Syst Rev. 2007 Apr 18;2007(2):MR000001. doi: 10.1002/14651858.MR000001.pub2. Cochrane Database Syst Rev. 2007. PMID: 17443625 Free PMC article. Review.
-
Identification and description of controlled clinical trials published in Spanish Ophthalmology Journals.Ophthalmic Epidemiol. 2018 Oct-Dec;25(5-6):436-442. doi: 10.1080/09286586.2018.1503688. Epub 2018 Aug 6. Ophthalmic Epidemiol. 2018. PMID: 30081705
-
Identification and description of controlled clinical trials published in Spanish Gynaecology and Obstetrics journals and risk of bias assessment of trials on assisted reproductive techniques.Eur J Obstet Gynecol Reprod Biol. 2016 Aug;203:5-11. doi: 10.1016/j.ejogrb.2016.04.039. Epub 2016 Apr 30. Eur J Obstet Gynecol Reprod Biol. 2016. PMID: 27235630 Review.
Cited by
-
A multiyear systematic survey of the quality of reporting for randomised trials in dentistry, neurology and geriatrics published in journals of Spain and Latin America.BMC Med Res Methodol. 2021 Jul 26;21(1):153. doi: 10.1186/s12874-021-01337-3. BMC Med Res Methodol. 2021. PMID: 34311704 Free PMC article.
-
Therapeutic use of cannabis and cannabinoids: an evidence mapping and appraisal of systematic reviews.BMC Complement Med Ther. 2020 Jan 15;20(1):12. doi: 10.1186/s12906-019-2803-2. BMC Complement Med Ther. 2020. PMID: 32020875 Free PMC article. Review.
References
-
- Johnell O, Kanis JA. An estimate of the worldwide prevalence, mortality and disability associated with hip fracture. Osteoporos Int 2004; 15(11): 897–902. - PubMed
-
- Johnell O, Kanis JA. An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos Int 2006; 17(12): 1726–1733. - PubMed
-
- Konnopka A, Jerusel N, Konig HH. The health and economic consequences of osteopenia- and osteoporosis-attributable hip fractures in Germany: estimation for 2002 and projection until 2050. Osteoporos Int 2009; 20(7): 1117–1129. - PubMed
-
- Morales-Torres J, Gutierrez-Urena S. The burden of osteoporosis in Latin America. Osteoporos Int 2004; 15(8): 625–632. - PubMed
-
- Sanders TL, Maradit Kremers H, Bryan AJ, et al. Incidence of anterior cruciate ligament tears and reconstruction: a 21-year population-based study. Am J Sports Med 2016; 44(6): 1502–1507. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

