Effects of 4-Week Intervention with Ulmus macrocarpa Hance Extract on Immune Function Biomarkers in Healthy Adults: A Randomized Controlled Trial
- PMID: 29681977
- PMCID: PMC5845525
- DOI: 10.1155/2018/5690816
Effects of 4-Week Intervention with Ulmus macrocarpa Hance Extract on Immune Function Biomarkers in Healthy Adults: A Randomized Controlled Trial
Abstract
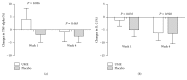
Ulmus macrocarpa extract has been shown to have immune-related effects in animals, but no studies have yet been performed in humans. This randomized, double-blind, placebo-controlled trial was conducted to determine the effect of short-term administration of Ulmus macrocarpa Hance extract (UME) on immune function biomarkers and its safety in human subjects. Fifty-eight subjects were randomly assigned to a UME group or a placebo group. Subjects in the UME group were given 500 mg per day of UME orally for 4 weeks. Mean fluorescence intensity (MFI) of tumor necrotic factor-α increased only in the UME group at 1 week (P = 0.027). The MFI of interleukin-2 decreased less significantly in the UME group than in the placebo group at 1 week (P = 0.028). However, unfortunately, at 4 weeks, no intergroup differences were detected in MFIs of cytokine. In conclusion, administration of UME for 1 week increased serum TNF-α and sustains IL-2 in human, which suggests that UME increases Th1-related immune function in the short term in healthy people. However, additional studies are needed to confirm the results of this first-stage study and further trials are required to decide on optimal dosage and duration of administration. This trial is registered with ClinicalTrials.gov Identifier: NCT02414412.
Figures
Similar articles
-
Potential lipid-lowering effects of Ulmus macrocarpa Hance extract in adults with untreated high low-density lipoprotein cholesterol concentrations: A randomized double-blind placebo-controlled trial.Front Med (Lausanne). 2022 Nov 1;9:1000428. doi: 10.3389/fmed.2022.1000428. eCollection 2022. Front Med (Lausanne). 2022. PMID: 36388925 Free PMC article.
-
Ulmus macrocarpa Hance extract modulates intestinal microbiota in healthy adults: a randomized, placebo-controlled clinical trial.J Microbiol. 2021 Dec;59(12):1150-1156. doi: 10.1007/s12275-021-1329-8. Epub 2021 Oct 26. J Microbiol. 2021. PMID: 34697783 Clinical Trial.
-
Extract of Ulmus macrocarpa Hance prevents thrombus formation through antiplatelet activity.Mol Med Rep. 2013 Sep;8(3):726-30. doi: 10.3892/mmr.2013.1581. Epub 2013 Jul 10. Mol Med Rep. 2013. PMID: 23846328
-
Folic acid with or without vitamin B12 for cognition and dementia.Cochrane Database Syst Rev. 2003;(4):CD004514. doi: 10.1002/14651858.CD004514. Cochrane Database Syst Rev. 2003. Update in: Cochrane Database Syst Rev. 2008 Oct 08;(4):CD004514. doi: 10.1002/14651858.CD004514.pub2. PMID: 14584018 Updated. Review.
-
The effect of vitamin B6 on cognition.Cochrane Database Syst Rev. 2003;(4):CD004393. doi: 10.1002/14651858.CD004393. Cochrane Database Syst Rev. 2003. PMID: 14584010 Review.
Cited by
-
Potential lipid-lowering effects of Ulmus macrocarpa Hance extract in adults with untreated high low-density lipoprotein cholesterol concentrations: A randomized double-blind placebo-controlled trial.Front Med (Lausanne). 2022 Nov 1;9:1000428. doi: 10.3389/fmed.2022.1000428. eCollection 2022. Front Med (Lausanne). 2022. PMID: 36388925 Free PMC article.
-
Ulmus macrocarpa Hance extract modulates intestinal microbiota in healthy adults: a randomized, placebo-controlled clinical trial.J Microbiol. 2021 Dec;59(12):1150-1156. doi: 10.1007/s12275-021-1329-8. Epub 2021 Oct 26. J Microbiol. 2021. PMID: 34697783 Clinical Trial.
References
-
- Kim K. S., Lee I. K. Clinical Evaluation of Immune-Promoting Functions of The Developed Product (HemoHIM) Korea Atomic Energy Research Institute; 2007.
-
- Lee S. J. Korean Folk Medicine, Monographs Series. Vol. 39. Seoul, Korea: Seoul National University Press; 1966. (Series No. 3).
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources

