Ciliary Motility: Regulation of Axonemal Dynein Motors
- PMID: 28765157
- PMCID: PMC5538414
- DOI: 10.1101/cshperspect.a018325
Ciliary Motility: Regulation of Axonemal Dynein Motors
Abstract
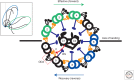
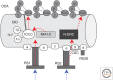
Ciliary motility is crucial for the development and health of many organisms. Motility depends on the coordinated activity of multiple dynein motors arranged in a precise pattern on the outer doublet microtubules. Although significant progress has been made in elucidating the composition and organization of the dyneins, a comprehensive understanding of dynein regulation is lacking. Here, we focus on two conserved signaling complexes located at the base of the radial spokes. These include the I1/f inner dynein arm associated with radial spoke 1 and the calmodulin- and spoke-associated complex and the nexin-dynein regulatory complex associated with radial spoke 2. Current research is focused on understanding how these two axonemal hubs coordinate and regulate the dynein motors and ciliary motility.
Copyright © 2017 Cold Spring Harbor Laboratory Press; all rights reserved.
Figures


Similar articles
-
The I1 dynein-associated tether and tether head complex is a conserved regulator of ciliary motility.Mol Biol Cell. 2018 May 1;29(9):1048-1059. doi: 10.1091/mbc.E18-02-0142. Epub 2018 Mar 22. Mol Biol Cell. 2018. PMID: 29514928 Free PMC article.
-
FAP57/WDR65 targets assembly of a subset of inner arm dyneins and connects to regulatory hubs in cilia.Mol Biol Cell. 2019 Oct 1;30(21):2659-2680. doi: 10.1091/mbc.E19-07-0367. Epub 2019 Sep 4. Mol Biol Cell. 2019. PMID: 31483737 Free PMC article.
-
Fifty years of microtubule sliding in cilia.Mol Biol Cell. 2018 Mar 15;29(6):698-701. doi: 10.1091/mbc.E17-07-0483. Mol Biol Cell. 2018. PMID: 29535180 Free PMC article.
-
Keeping an eye on I1: I1 dynein as a model for flagellar dynein assembly and regulation.Cell Motil Cytoskeleton. 2007 Aug;64(8):569-79. doi: 10.1002/cm.20211. Cell Motil Cytoskeleton. 2007. PMID: 17549744 Review.
-
Ciliary dynein arms: Cytoplasmic preassembly, intraflagellar transport, and axonemal docking.J Cell Physiol. 2022 Jun;237(6):2644-2653. doi: 10.1002/jcp.30689. Epub 2022 Feb 6. J Cell Physiol. 2022. PMID: 35128656 Review.
Cited by
-
Ammonia Exposure Induced Cilia Dysfunction of Nasal Mucosa in the Piglets.Biomed Res Int. 2020 May 25;2020:1705387. doi: 10.1155/2020/1705387. eCollection 2020. Biomed Res Int. 2020. PMID: 32566662 Free PMC article.
-
The Increase in the Frequency and Amplitude of the Beating of Isolated Mouse Tracheal Cilia Reactivated by ATP and cAMP with Elevation in pH.Int J Mol Sci. 2024 Jul 26;25(15):8138. doi: 10.3390/ijms25158138. Int J Mol Sci. 2024. PMID: 39125708 Free PMC article.
-
Structural organization of the intermediate and light chain complex of Chlamydomonas ciliary I1 dynein.FASEB J. 2021 Jun;35(6):e21646. doi: 10.1096/fj.202001857R. FASEB J. 2021. PMID: 33993568 Free PMC article.
-
Rare Human Diseases: Model Organisms in Deciphering the Molecular Basis of Primary Ciliary Dyskinesia.Cells. 2019 Dec 11;8(12):1614. doi: 10.3390/cells8121614. Cells. 2019. PMID: 31835861 Free PMC article. Review.
-
Microtubules and Microtubule-Associated Proteins.Cold Spring Harb Perspect Biol. 2018 Jun 1;10(6):a022608. doi: 10.1101/cshperspect.a022608. Cold Spring Harb Perspect Biol. 2018. PMID: 29858272 Free PMC article. Review.
References
-
- Austin-Tse C, Halbritter J, Zariwala MA, Gilberti RM, Gee HY, Hellman N, Pathak N, Liu Y, Panizzi JR, Patel-King RS, et al. 2013. Zebrafish ciliopathy screen plus human mutational analysis identifies C21orf59 and CCDC65 defects as causing primary ciliary dyskinesia. Am J Hum Genet 93: 672–686. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
