Progress in Understanding the Molecular Basis Underlying Functional Diversification of Cyclic Dinucleotide Turnover Proteins
- PMID: 28031279
- PMCID: PMC5309910
- DOI: 10.1128/JB.00790-16
Progress in Understanding the Molecular Basis Underlying Functional Diversification of Cyclic Dinucleotide Turnover Proteins
Abstract
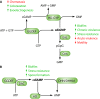
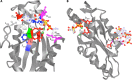
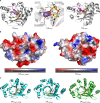
Cyclic di-GMP was the first cyclic dinucleotide second messenger described, presaging the discovery of additional cyclic dinucleotide messengers in bacteria and eukaryotes. The GGDEF diguanylate cyclase (DGC) and EAL and HD-GYP phosphodiesterase (PDE) domains conduct the turnover of cyclic di-GMP. These three unrelated domains belong to superfamilies that exhibit significant variations in function, and they include both enzymatically active and inactive members, with a subset involved in synthesis and degradation of other cyclic dinucleotides. Here, we summarize current knowledge of sequence and structural variations that underpin the functional diversification of cyclic di-GMP turnover proteins. Moreover, we highlight that superfamily diversification is not restricted to cyclic di-GMP signaling domains, as particular DHH/DHHA1 domain and HD domain proteins have been shown to act as cyclic di-AMP phosphodiesterases. We conclude with a consideration of the current limitations that such diversity of action places on bioinformatic prediction of the roles of GGDEF, EAL, and HD-GYP domain proteins.
Keywords: DHH/DHHA1 domain; EAL domain; GGDEF domain; HD-GYP domain; cyclic GAMP; cyclic di-AMP; cyclic di-GMP; cyclic dinucleotide second messenger; second messenger.
Copyright © 2017 American Society for Microbiology.
Figures


Similar articles
-
More than Enzymes That Make or Break Cyclic Di-GMP-Local Signaling in the Interactome of GGDEF/EAL Domain Proteins of Escherichia coli.mBio. 2017 Oct 10;8(5):e01639-17. doi: 10.1128/mBio.01639-17. mBio. 2017. PMID: 29018125 Free PMC article.
-
The structure of an unconventional HD-GYP protein from Bdellovibrio reveals the roles of conserved residues in this class of cyclic-di-GMP phosphodiesterases.mBio. 2011 Oct 11;2(5):e00163-11. doi: 10.1128/mBio.00163-11. Print 2011. mBio. 2011. PMID: 21990613 Free PMC article.
-
Identification and characterization of a cyclic di-GMP-specific phosphodiesterase and its allosteric control by GTP.J Biol Chem. 2005 Sep 2;280(35):30829-37. doi: 10.1074/jbc.M504429200. Epub 2005 Jul 1. J Biol Chem. 2005. PMID: 15994307
-
The HD-GYP domain, cyclic di-GMP signaling, and bacterial virulence to plants.Mol Plant Microbe Interact. 2006 Dec;19(12):1378-84. doi: 10.1094/MPMI-19-1378. Mol Plant Microbe Interact. 2006. PMID: 17153922 Review.
-
Cyclic di-GMP signalling and the regulation of bacterial virulence.Microbiology (Reading). 2013 Jul;159(Pt 7):1286-1297. doi: 10.1099/mic.0.068189-0. Epub 2013 May 23. Microbiology (Reading). 2013. PMID: 23704785 Free PMC article. Review.
Cited by
-
Comparative genomics analysis of c-di-GMP metabolism and regulation in Microcystis aeruginosa.BMC Genomics. 2020 Mar 9;21(1):217. doi: 10.1186/s12864-020-6591-3. BMC Genomics. 2020. PMID: 32151246 Free PMC article.
-
Identification of c-di-GMP Signaling Components in Xanthomonas oryzae and Their Orthologs in Xanthomonads Involved in Regulation of Bacterial Virulence Expression.Front Microbiol. 2019 Jul 11;10:1402. doi: 10.3389/fmicb.2019.01402. eCollection 2019. Front Microbiol. 2019. PMID: 31354637 Free PMC article. Review.
-
Identification of Three New GGDEF and EAL Domain-Containing Proteins Participating in the Scr Surface Colonization Regulatory Network in Vibrio parahaemolyticus.J Bacteriol. 2021 Jan 25;203(4):e00409-20. doi: 10.1128/JB.00409-20. Print 2021 Jan 25. J Bacteriol. 2021. PMID: 33199284 Free PMC article.
-
Physiological and Microscopic Characterization of Cyclic-di-GMP-Mediated Autoaggregation in Erwinia amylovora.Front Microbiol. 2019 Mar 12;10:468. doi: 10.3389/fmicb.2019.00468. eCollection 2019. Front Microbiol. 2019. PMID: 30930874 Free PMC article.
-
CdgC, a Cyclic-di-GMP Diguanylate Cyclase of Azospirillum baldaniorum Is Involved in Internalization to Wheat Roots.Front Plant Sci. 2021 Oct 20;12:748393. doi: 10.3389/fpls.2021.748393. eCollection 2021. Front Plant Sci. 2021. PMID: 34745182 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

