Germline variants in ETV6 underlie reduced platelet formation, platelet dysfunction and increased levels of circulating CD34+ progenitors
- PMID: 27663637
- PMCID: PMC5286936
- DOI: 10.3324/haematol.2016.147694
Germline variants in ETV6 underlie reduced platelet formation, platelet dysfunction and increased levels of circulating CD34+ progenitors
Abstract
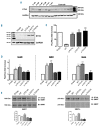
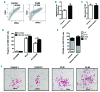
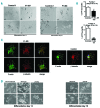
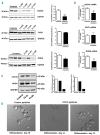
Variants in ETV6, which encodes a transcription repressor of the E26 transformation-specific family, have recently been reported to be responsible for inherited thrombocytopenia and hematologic malignancy. We sequenced the DNA from cases with unexplained dominant thrombocytopenia and identified six likely pathogenic variants in ETV6, of which five are novel. We observed low repressive activity of all tested ETV6 variants, and variants located in the E26 transformation-specific binding domain (encoding p.A377T, p.Y401N) led to reduced binding to corepressors. We also observed a large expansion of megakaryocyte colony-forming units derived from variant carriers and reduced proplatelet formation with abnormal cytoskeletal organization. The defect in proplatelet formation was also observed in control CD34+ cell-derived megakaryocytes transduced with lentiviral particles encoding mutant ETV6. Reduced expression levels of key regulators of the actin cytoskeleton CDC42 and RHOA were measured. Moreover, changes in the actin structures are typically accompanied by a rounder platelet shape with a highly heterogeneous size, decreased platelet arachidonic response, and spreading and retarded clot retraction in ETV6 deficient platelets. Elevated numbers of circulating CD34+ cells were found in p.P214L and p.Y401N carriers, and two patients from different families suffered from refractory anemia with excess blasts, while one patient from a third family was successfully treated for acute myeloid leukemia. Overall, our study provides novel insights into the role of ETV6 as a driver of cytoskeletal regulatory gene expression during platelet production, and the impact of variants resulting in platelets with altered size, shape and function and potentially also in changes in circulating progenitor levels.
Copyright© Ferrata Storti Foundation.
Figures


Similar articles
-
Germline ETV6 mutations and predisposition to hematological malignancies.Int J Hematol. 2017 Aug;106(2):189-195. doi: 10.1007/s12185-017-2259-4. Epub 2017 May 29. Int J Hematol. 2017. PMID: 28555414 Review.
-
Clinical and pathogenic features of ETV6-related thrombocytopenia with predisposition to acute lymphoblastic leukemia.Haematologica. 2016 Nov;101(11):1333-1342. doi: 10.3324/haematol.2016.147496. Epub 2016 Jun 30. Haematologica. 2016. PMID: 27365488 Free PMC article.
-
Single-cell analysis of megakaryopoiesis in peripheral CD34+ cells: insights into ETV6-related thrombocytopenia.J Thromb Haemost. 2023 Sep;21(9):2528-2544. doi: 10.1016/j.jtha.2023.04.007. Epub 2023 Apr 20. J Thromb Haemost. 2023. PMID: 37085035
-
Study of inherited thrombocytopenia resulting from mutations in ETV6 or RUNX1 using a human pluripotent stem cell model.Stem Cell Reports. 2021 Jun 8;16(6):1458-1467. doi: 10.1016/j.stemcr.2021.04.013. Epub 2021 May 20. Stem Cell Reports. 2021. PMID: 34019812 Free PMC article.
-
ETV6 in hematopoiesis and leukemia predisposition.Semin Hematol. 2017 Apr;54(2):98-104. doi: 10.1053/j.seminhematol.2017.04.005. Epub 2017 Apr 7. Semin Hematol. 2017. PMID: 28637624 Free PMC article. Review.
Cited by
-
Toward prevention of childhood ALL by early-life immune training.Blood. 2021 Oct 21;138(16):1412-1428. doi: 10.1182/blood.2020009895. Blood. 2021. PMID: 34010407 Free PMC article.
-
Genetic predisposition to MDS: clinical features and clonal evolution.Blood. 2019 Mar 7;133(10):1071-1085. doi: 10.1182/blood-2018-10-844662. Epub 2019 Jan 22. Blood. 2019. PMID: 30670445 Free PMC article. Review.
-
Megakaryopoiesis and Platelet Biology: Roles of Transcription Factors and Emerging Clinical Implications.Int J Mol Sci. 2021 Sep 5;22(17):9615. doi: 10.3390/ijms22179615. Int J Mol Sci. 2021. PMID: 34502524 Free PMC article. Review.
-
Germline ETV6 mutations and predisposition to hematological malignancies.Int J Hematol. 2017 Aug;106(2):189-195. doi: 10.1007/s12185-017-2259-4. Epub 2017 May 29. Int J Hematol. 2017. PMID: 28555414 Review.
-
Genetic Predisposition to Myelodysplastic Syndrome in Clinical Practice.Hematol Oncol Clin North Am. 2020 Apr;34(2):333-356. doi: 10.1016/j.hoc.2019.10.002. Epub 2020 Jan 6. Hematol Oncol Clin North Am. 2020. PMID: 32089214 Free PMC article. Review.
References
-
- Rowley JD. The critical role of chromosome translocations in human leukemias. Annu Rev Genet. 1998;32:495–519. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

