Wnt signaling in cancer
- PMID: 27617575
- PMCID: PMC5357762
- DOI: 10.1038/onc.2016.304
Wnt signaling in cancer
Abstract
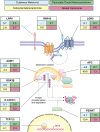
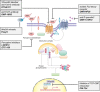
Wnt signaling is one of the key cascades regulating development and stemness, and has also been tightly associated with cancer. The role of Wnt signaling in carcinogenesis has most prominently been described for colorectal cancer, but aberrant Wnt signaling is observed in many more cancer entities. Here, we review current insights into novel components of Wnt pathways and describe their impact on cancer development. Furthermore, we highlight expanding functions of Wnt signaling for both solid and liquid tumors. We also describe current findings how Wnt signaling affects maintenance of cancer stem cells, metastasis and immune control. Finally, we provide an overview of current strategies to antagonize Wnt signaling in cancer and challenges that are associated with such approaches.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Targeting the Wnt/β-catenin signaling pathway in cancer.J Hematol Oncol. 2020 Dec 4;13(1):165. doi: 10.1186/s13045-020-00990-3. J Hematol Oncol. 2020. PMID: 33276800 Free PMC article. Review.
-
The involvement of noncanonical Wnt signaling in cancers.Biomed Pharmacother. 2021 Jan;133:110946. doi: 10.1016/j.biopha.2020.110946. Epub 2020 Nov 16. Biomed Pharmacother. 2021. PMID: 33212376 Review.
-
Targeting the Versatile Wnt/β-Catenin Pathway in Cancer Biology and Therapeutics: From Concept to Actionable Strategy.OMICS. 2019 Nov;23(11):517-538. doi: 10.1089/omi.2019.0147. Epub 2019 Oct 15. OMICS. 2019. PMID: 31613700
-
Wnt/beta-catenin pathway: modulating anticancer immune response.J Hematol Oncol. 2017 May 5;10(1):101. doi: 10.1186/s13045-017-0471-6. J Hematol Oncol. 2017. PMID: 28476164 Free PMC article. Review.
-
Compound Kushen Injection suppresses human breast cancer stem-like cells by down-regulating the canonical Wnt/β-catenin pathway.J Exp Clin Cancer Res. 2011 Oct 28;30(1):103. doi: 10.1186/1756-9966-30-103. J Exp Clin Cancer Res. 2011. PMID: 22032476 Free PMC article.
Cited by
-
Crosstalk between the Hippo Pathway and the Wnt Pathway in Huntington's Disease and Other Neurodegenerative Disorders.Cells. 2022 Nov 16;11(22):3631. doi: 10.3390/cells11223631. Cells. 2022. PMID: 36429058 Free PMC article. Review.
-
Ethylparaben induces subconjunctival fibrosis via the Wnt/β-catenin signaling pathway.Exp Ther Med. 2021 Apr;21(4):295. doi: 10.3892/etm.2021.9726. Epub 2021 Jan 28. Exp Ther Med. 2021. PMID: 33717238 Free PMC article.
-
Linking GOLPH3 and Extracellular Vesicles Content-a Potential New Route in Cancer Physiopathology and a Promising Therapeutic Target is in Sight?Technol Cancer Res Treat. 2022 Jan-Dec;21:15330338221135724. doi: 10.1177/15330338221135724. Technol Cancer Res Treat. 2022. PMID: 36320176 Free PMC article. Review.
-
Dickkopf-1: A Promising Target for Cancer Immunotherapy.Front Immunol. 2021 May 20;12:658097. doi: 10.3389/fimmu.2021.658097. eCollection 2021. Front Immunol. 2021. PMID: 34093545 Free PMC article. Review.
-
An In Silico Analysis Identified FZD9 as a Potential Prognostic Biomarker in Triple-Negative Breast Cancer Patients.Eur J Breast Health. 2020 Dec 24;17(1):42-52. doi: 10.4274/ejbh.2020.5804. eCollection 2021 Jan. Eur J Breast Health. 2020. PMID: 33796830 Free PMC article.
References
-
- Sharma R. Wingless, a new mutant in D. melanogaster. Drosoph Inf Serv 1973; 50: 134.
-
- Nüsslein-Volhard C, Wieschaus E. Mutations affecting segment number and polarity in Drosophila. Nature 1980; 287: 795–801. - PubMed
-
- Tsukamoto AS, Grosschedl R, Guzman RC, Parslow T, Varmus HE. Expression of the int-1 gene in transgenic mice is associated with mammary gland hyperplasia and adenocarcinomas in male and female mice. Cell 1988; 55: 619–625. - PubMed
-
- Nusse R, van Ooyen A, Cox D, Fung YK, Varmus H. Mode of proviral activation of a putative mammary oncogene (int-1) on mouse chromosome 15. Nature 307: 131–136. - PubMed
-
- Nusse R, Varmus HE. Many tumors induced by the mouse mammary tumor virus contain a provirus integrated in the same region of the host genome. Cell 1982; 31: 99–109. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

