Group B Streptococcus Degrades Cyclic-di-AMP to Modulate STING-Dependent Type I Interferon Production
- PMID: 27414497
- PMCID: PMC5382021
- DOI: 10.1016/j.chom.2016.06.003
Group B Streptococcus Degrades Cyclic-di-AMP to Modulate STING-Dependent Type I Interferon Production
Abstract
Induction of type I interferon (IFN) in response to microbial pathogens depends on a conserved cGAS-STING signaling pathway. The presence of DNA in the cytoplasm activates cGAS, while STING is activated by cyclic dinucleotides (cdNs) produced by cGAS or from bacterial origins. Here, we show that Group B Streptococcus (GBS) induces IFN-β production almost exclusively through cGAS-STING-dependent recognition of bacterial DNA. However, we find that GBS expresses an ectonucleotidase, CdnP, which hydrolyzes extracellular bacterial cyclic-di-AMP. Inactivation of CdnP leads to c-di-AMP accumulation outside the bacteria and increased IFN-β production. Higher IFN-β levels in vivo increase GBS killing by the host. The IFN-β overproduction observed in the absence of CdnP is due to the cumulative effect of DNA sensing by cGAS and STING-dependent sensing of c-di-AMP. These findings describe the importance of a bacterial c-di-AMP ectonucleotidase and suggest a direct bacterial mechanism that dampens activation of the cGAS-STING axis.
Keywords: Streptococcus agalactiae; c-di-AMP; cGAS; ectonucleotidase; interferon-β.
Copyright © 2016 Elsevier Inc. All rights reserved.
Figures


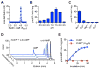

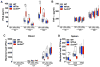

Comment in
-
Flying Under the Radar: Immune Evasion by Group B Streptococcus.Cell Host Microbe. 2016 Jul 13;20(1):4-6. doi: 10.1016/j.chom.2016.06.015. Cell Host Microbe. 2016. PMID: 27414494
Similar articles
-
The analog of cGAMP, c-di-AMP, activates STING mediated cell death pathway in estrogen-receptor negative breast cancer cells.Apoptosis. 2021 Jun;26(5-6):293-306. doi: 10.1007/s10495-021-01669-x. Epub 2021 Apr 10. Apoptosis. 2021. PMID: 33840002
-
STING-dependent recognition of cyclic di-AMP mediates type I interferon responses during Chlamydia trachomatis infection.mBio. 2013 Apr 30;4(3):e00018-13. doi: 10.1128/mBio.00018-13. mBio. 2013. PMID: 23631912 Free PMC article.
-
A bacterial cyclic dinucleotide activates the cytosolic surveillance pathway and mediates innate resistance to tuberculosis.Nat Med. 2015 Apr;21(4):401-6. doi: 10.1038/nm.3813. Epub 2015 Mar 2. Nat Med. 2015. PMID: 25730264 Free PMC article.
-
Cyclic di-AMP in host-pathogen interactions.Curr Opin Microbiol. 2018 Feb;41:21-28. doi: 10.1016/j.mib.2017.11.007. Epub 2017 Nov 21. Curr Opin Microbiol. 2018. PMID: 29169058 Review.
-
Interrupting cyclic dinucleotide-cGAS-STING axis with small molecules.Medchemcomm. 2019 Aug 15;10(12):1999-2023. doi: 10.1039/c8md00555a. eCollection 2019 Dec 1. Medchemcomm. 2019. PMID: 32206239 Free PMC article. Review.
Cited by
-
Leishmania kinetoplast DNA contributes to parasite burden in infected macrophages: Critical role of the cGAS-STING-TBK1 signaling pathway in macrophage parasitemia.Front Immunol. 2022 Nov 2;13:1007070. doi: 10.3389/fimmu.2022.1007070. eCollection 2022. Front Immunol. 2022. PMID: 36405710 Free PMC article.
-
Increased Intracellular Cyclic di-AMP Levels Sensitize Streptococcus gallolyticus subsp. gallolyticus to Osmotic Stress and Reduce Biofilm Formation and Adherence on Intestinal Cells.J Bacteriol. 2019 Feb 25;201(6):e00597-18. doi: 10.1128/JB.00597-18. Print 2019 Mar 15. J Bacteriol. 2019. PMID: 30617242 Free PMC article.
-
Effect of titanium implants along with silver ions and tetracycline on type I interferon-beta expression during implant-related infections in co-culture and mouse model.Front Bioeng Biotechnol. 2023 Oct 19;11:1227148. doi: 10.3389/fbioe.2023.1227148. eCollection 2023. Front Bioeng Biotechnol. 2023. PMID: 37929187 Free PMC article.
-
An opportunistic pathogen under stress: how Group B Streptococcus responds to cytotoxic reactive species and conditions of metal ion imbalance to survive.FEMS Microbiol Rev. 2024 May 8;48(3):fuae009. doi: 10.1093/femsre/fuae009. FEMS Microbiol Rev. 2024. PMID: 38678005 Free PMC article. Review.
-
Opposing roles of Toll-like receptor and cytosolic DNA-STING signaling pathways for Staphylococcus aureus cutaneous host defense.PLoS Pathog. 2017 Jul 13;13(7):e1006496. doi: 10.1371/journal.ppat.1006496. eCollection 2017 Jul. PLoS Pathog. 2017. PMID: 28704551 Free PMC article.
References
-
- Anraku Y. A New Cyclic Phosphodiesterase Having a 3′-Nucleotidase Activity from Escherichia Coli B. II. Further Studies on Substrate Specificity and Mode of Action of the Enzyme. J Biol Chem. 1964;239:3420–3424. - PubMed
-
- Barber GN. STING-dependent cytosolic DNA sensing pathways. Trends in immunology. 2014;35:88–93. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

