Twenty Years of Mycobacterial Glycans: Furanosides and Beyond
- PMID: 27294709
- PMCID: PMC4955529
- DOI: 10.1021/acs.accounts.6b00164
Twenty Years of Mycobacterial Glycans: Furanosides and Beyond
Abstract
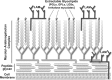
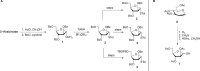
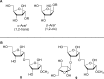
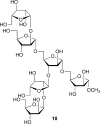
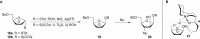
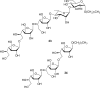
The cell surface (or cell wall) of bacteria is coated with carbohydrate (or glycan) structures that play a number of important roles. These include providing structural integrity, serving as a permeability barrier to extracellular compounds (e.g., drugs) and modulating the immune system of the host. Of interest to this Account is the cell wall structure of mycobacteria. There are a host of different mycobacterial species, some of which cause human disease. The most well-known is Mycobacterium tuberculosis, the causative agent of tuberculosis. The mycobacterial cell wall is characterized by the presence of unusual carbohydrate structures that fulfill the roles described above. However, in many cases, a molecular-level understanding of how mycobacterial cell wall glycans mediate these processes is lacking. Inspired by a seminar he heard as a postdoctoral fellow, the author began his independent research program with a focus on the chemical synthesis of mycobacterial glycans. The goals were not only to develop synthetic approaches to these unique structures but also to provide molecules that could be used to probe their biological function. Initial work addressed the preparation of fragments of two key polysaccharides, arabinogalactan and lipoarabinomannan, which contain large numbers of sugar residues in the furanose (five-membered) ring form. At the time these investigations began, there were few methods reported for the synthesis of oligosaccharides containing furanose rings. Thus, early in the program, a major area of interest was methodology development, particularly for the preparation of 1,2-cis-furanosides. To solve this challenge, a range of conformationally restricted donors have been developed, both in the author's group and others, which provide 1,2-cis-furanosidic linkages with high stereoselectivity. These investigations were followed by application of the developed methods to the synthesis of a range of target molecules containing arabinofuranose and galactofuranose residues. These molecules have now found application in biochemical, immunological, and structural biology investigations, which have shed light on their biosynthesis and how these motifs are recognized by both the innate and adaptive immune systems. More recently, attention has been directed toward the synthesis of another class of immunologically active mycobacterial cell wall glycans, the extractable glycolipids. In this case, efforts have been primarily on phenolic glycolipids, and the compounds synthesized have been used to evaluate their ability to modulate cytokine release. Over the past 20 years, the use of chemical synthesis to provide increasingly complex glycan structures has provided significant benefit to the burgeoning field of mycobacterial glycobiology. Through the efforts of groups from around the globe, access to these compounds is now possible via relatively straightforward methods. As the pool of mycobacterial glycans continues to grow, so too will our understanding of their role in disease, which will undoubtedly lead to new strategies to prevent or treat mycobacterial infections.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


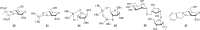
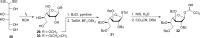

Similar articles
-
Insights into Interactions of Mycobacteria with the Host Innate Immune System from a Novel Array of Synthetic Mycobacterial Glycans.ACS Chem Biol. 2017 Dec 15;12(12):2990-3002. doi: 10.1021/acschembio.7b00797. Epub 2017 Nov 3. ACS Chem Biol. 2017. PMID: 29048873 Free PMC article.
-
Synthesis of the docosanasaccharide arabinan domain of mycobacterial arabinogalactan and a proposed octadecasaccharide biosynthetic precursor.J Am Chem Soc. 2007 Aug 15;129(32):9885-901. doi: 10.1021/ja072892+. Epub 2007 Jul 26. J Am Chem Soc. 2007. PMID: 17655235
-
Automated Glycan Assembly of Mycobacterial Hexaarabinofuranoside and Docosasaccharide Arabinan (Araf23 ) Motifs found on Mycobacterium tuberculosis.Chemistry. 2023 Apr 21;29(23):e202300032. doi: 10.1002/chem.202300032. Epub 2023 Mar 16. Chemistry. 2023. PMID: 36745435
-
[Development of antituberculous drugs: current status and future prospects].Kekkaku. 2006 Dec;81(12):753-74. Kekkaku. 2006. PMID: 17240921 Review. Japanese.
-
The Emergence of Phenolic Glycans as Virulence Factors in Mycobacterium tuberculosis.ACS Chem Biol. 2017 Aug 18;12(8):1969-1979. doi: 10.1021/acschembio.7b00394. Epub 2017 Jul 19. ACS Chem Biol. 2017. PMID: 28692249 Review.
Cited by
-
An SN2-Type Strategy toward 1,2-cis-Furanosides.CCS Chem. 2022 Dec;4(12):3677-3685. doi: 10.31635/ccschem.022.202202175. Epub 2022 Dec 7. CCS Chem. 2022. PMID: 38186678 Free PMC article.
-
Why is thiol unexpectedly less reactive but more selective than alcohol in phenanthroline-catalyzed 1,2-cis O- and S-furanosylations?Org Biomol Chem. 2025 Jan 2;23(2):328-342. doi: 10.1039/d4ob01593b. Org Biomol Chem. 2025. PMID: 39575458 Free PMC article.
-
Total synthesis of mycobacterial arabinogalactan containing 92 monosaccharide units.Nat Commun. 2017 Mar 16;8:14851. doi: 10.1038/ncomms14851. Nat Commun. 2017. PMID: 28300074 Free PMC article.
-
An ultra-low thiourea catalyzed strain-release glycosylation and a multicatalytic diversification strategy.Nat Commun. 2018 Oct 3;9(1):4057. doi: 10.1038/s41467-018-06329-4. Nat Commun. 2018. PMID: 30282986 Free PMC article.
-
Computational and NMR Conformational Analysis of Galactofuranoside Cycles Presented in Bacterial and Fungal Polysaccharide Antigens.Front Mol Biosci. 2021 Aug 25;8:719396. doi: 10.3389/fmolb.2021.719396. eCollection 2021. Front Mol Biosci. 2021. PMID: 34513924 Free PMC article.
References
-
- Brennan P. J.ILC2.1 The Surface Structures of Mycobacteria: Their Roles in the Host Response, Their Biogenesis and Potential As Drug Targets, Presented at the 17th International Carbohydrate Symposium, Ottawa, ON Canada July17–22, 1994.
-
- Bai B.; Chu C.; Lowary T. Lipooligosaccharides from Mycobacteria: Structure, Function, and Synthesis. Isr. J. Chem. 2015, 55, 360–372. 10.1002/ijch.201400194. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

