Loss of GltB Inhibits Biofilm Formation and Biocontrol Efficiency of Bacillus subtilis Bs916 by Altering the Production of γ-Polyglutamate and Three Lipopeptides
- PMID: 27223617
- PMCID: PMC4880196
- DOI: 10.1371/journal.pone.0156247
Loss of GltB Inhibits Biofilm Formation and Biocontrol Efficiency of Bacillus subtilis Bs916 by Altering the Production of γ-Polyglutamate and Three Lipopeptides
Abstract
Aims: This study examined the contribution of GltB on biofilm formation and biocontrol efficiency of B. subtilis Bs916.
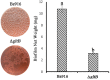
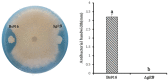
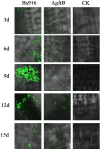
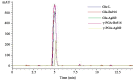
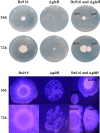
Methods and results: The gltB gene was identified through a biofilm phenotype screen and a bioinformatics analysis of serious biofilm formation defects, and then a gltB single knockout mutant was constructed using homologous recombination. This mutant demonstrated severe deficits in biofilm formation and colonisation along with significantly altered production ofγ-polyglutamate (γ-PGA) and three lipopeptide antibiotics (LPs) as measured by a transcriptional analysis of both the wild type B. subtilis Bs916 and the gltB mutant. Consequently, the mutant strain retained almost no antifungal activity against Rhizoctonia solani and exhibited decreased biocontrol efficiency against rice sheath blight. Very few gltB mutant cells colonised the rice stem, and they exhibited no significant nutrient chemotaxis compared to the wild type B. subtilis Bs916. The mechanism underlying these deficits in the gltB mutant appears to be decreased significantly in production of γ-PGA and a reduction in the production of both bacillomycin L and fengycin. Biofilm restoration of gltB mutant by additionγ-PGA in the EM medium demonstrated that biofilm formation was able to restore significantly at 20 g/L.
Conclusions: GltB regulates biofilm formation by altering the production ofγ-PGA, the LPs bacillomycin L and fengcin and influences bacterial colonisation on the rice stem, which consequently leads to poor biocontrol efficiency against rice sheath blight.
Significance and impact of study: This is the first report of a key regulatory protein (GltB) that is involved in biofilm regulation and its regulation mechanism and biocontrol efficiency by B. subtilis.
Conflict of interest statement
Figures

Similar articles
-
Bacillus subtilis biofilm formation and social interactions.Nat Rev Microbiol. 2021 Sep;19(9):600-614. doi: 10.1038/s41579-021-00540-9. Epub 2021 Apr 6. Nat Rev Microbiol. 2021. PMID: 33824496 Review.
-
Bacillomycin L and surfactin contribute synergistically to the phenotypic features of Bacillus subtilis 916 and the biocontrol of rice sheath blight induced by Rhizoctonia solani.Appl Microbiol Biotechnol. 2015 Feb;99(4):1897-910. doi: 10.1007/s00253-014-6195-4. Epub 2014 Nov 16. Appl Microbiol Biotechnol. 2015. PMID: 25398282
-
DegQ regulates the production of fengycins and biofilm formation of the biocontrol agent Bacillus subtilis NCD-2.Microbiol Res. 2015 Sep;178:42-50. doi: 10.1016/j.micres.2015.06.006. Epub 2015 Jul 2. Microbiol Res. 2015. PMID: 26302846
-
Genetic variants of the oppA gene are involved in metabolic regulation of surfactin in Bacillus subtilis.Microb Cell Fact. 2019 Aug 19;18(1):141. doi: 10.1186/s12934-019-1176-z. Microb Cell Fact. 2019. PMID: 31426791 Free PMC article.
-
Biological activity of lipopeptides from Bacillus.Appl Microbiol Biotechnol. 2017 Aug;101(15):5951-5960. doi: 10.1007/s00253-017-8396-0. Epub 2017 Jul 6. Appl Microbiol Biotechnol. 2017. PMID: 28685194 Review.
Cited by
-
Bacillus subtilis biofilm formation and social interactions.Nat Rev Microbiol. 2021 Sep;19(9):600-614. doi: 10.1038/s41579-021-00540-9. Epub 2021 Apr 6. Nat Rev Microbiol. 2021. PMID: 33824496 Review.
-
In vitro Activity of Cefepime/Avibactam Against Carbapenem Resistant Klebsiella pneumoniae and Integrative Metabolomics-Proteomics Approach for Resistance Mechanism: A Single-Center Study.Infect Drug Resist. 2023 Sep 11;16:6061-6077. doi: 10.2147/IDR.S420898. eCollection 2023. Infect Drug Resist. 2023. PMID: 37719649 Free PMC article.
-
Strategies for improving fengycin production: a review.Microb Cell Fact. 2024 May 22;23(1):144. doi: 10.1186/s12934-024-02425-x. Microb Cell Fact. 2024. PMID: 38773450 Free PMC article. Review.
-
Two New Biocontrol Agents Against Clubroot Caused by Plasmodiophora brassicae.Front Microbiol. 2020 Jan 21;10:3099. doi: 10.3389/fmicb.2019.03099. eCollection 2019. Front Microbiol. 2020. PMID: 32038545 Free PMC article.
-
Should the biofilm mode of life be taken into consideration for microbial biocontrol agents?Microb Biotechnol. 2017 Jul;10(4):719-734. doi: 10.1111/1751-7915.12693. Epub 2017 Feb 16. Microb Biotechnol. 2017. PMID: 28205337 Free PMC article. Review.
References
-
- O'Toole GA, Pratt LA, Watnick PI, et al. Genetic approaches to study of biofilms. Methods in enzymology, 1999; 310: 91–109. ISBN 9780121822118 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

